Immunogenic compositions and uses thereof
a technology of compositions and compositions, applied in the field of0003nucleic acid based vaccines, can solve the problems of uncontrollable undesirable sustained expression of antigens, and inability to rule out the risk of uncontrolled propagation of introduced genes and viral genes, so as to enhance the immune response to the pathogen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
stration of RNA and Protein to Mice
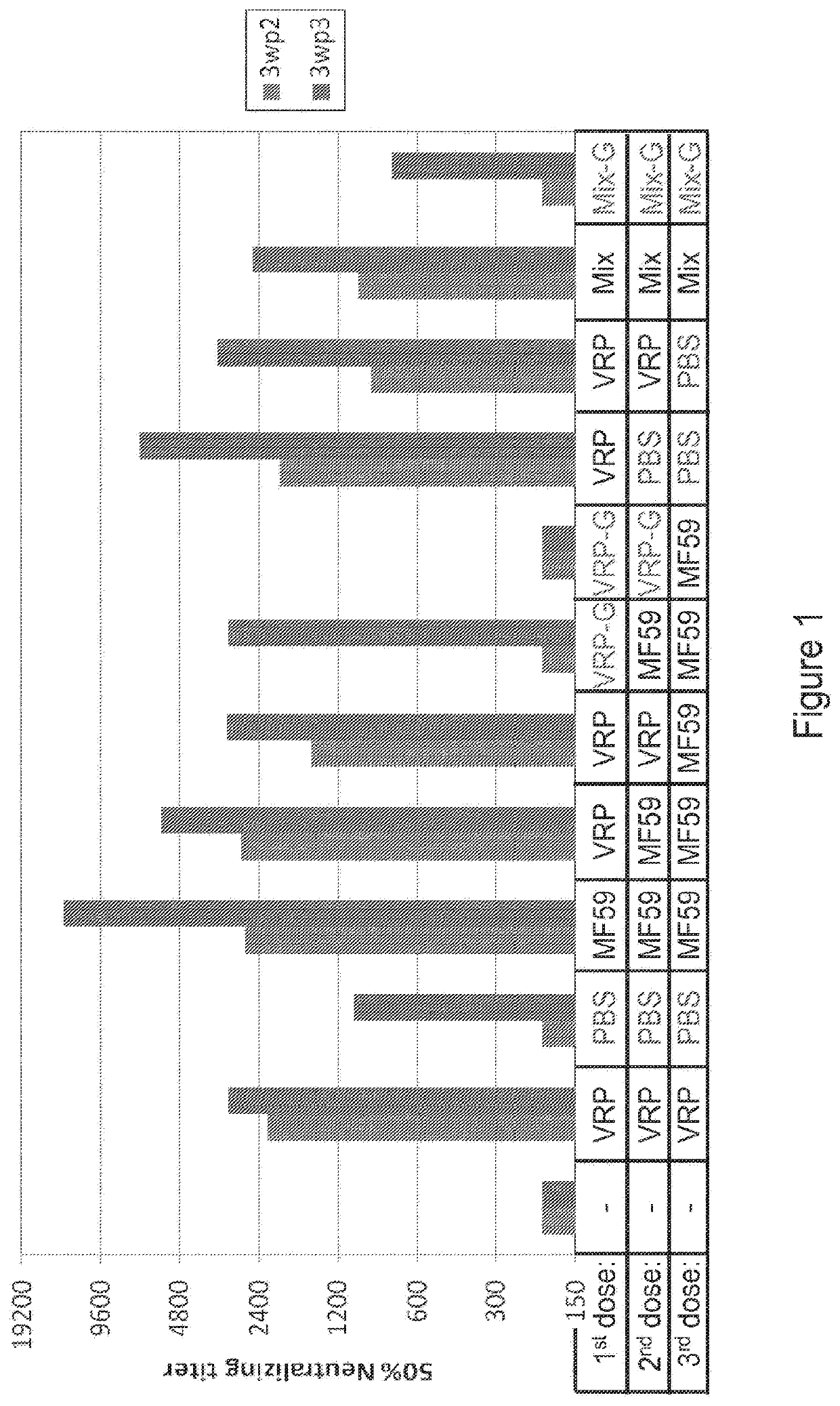
[0418]In this example, an RNA molecule encoding the RSV-F, or an RNA molecule encoding the GFP protein, was co-administered with an RSV-F antigen in polypeptide form. The effects of RNA on its “cognate” antigen and “non-cognate” antigen were assessed. The RSV-F antigen is a “non-cognate” antigen of the GFP-coding RNA because the F antigen does not share sequence homology to, and does not immunologically cross-react with the polypeptide encoded by the RNA molecule (GFP).
[0419]Three RNAs were used for this study: the vA317 replicon that expresses the surface fusion glycoprotein of RSV (RSV-F); the vA17 replicon that expresses green fluorescent protein (GFP); and the vA336 replicon that is replication-defective and encodes GFP. BALB / c mice, 5 animals per group, were given bilateral intramuscular vaccinations (50 μL per leg) on days 0 and 21. Spleens were harvested at day 49 for T cell analysis. Animals, 70 total, were divided into 14 groups (5 animals...
example ii
tration of RNA and Protein and Sequential Administration of RNA and Protein to Mice
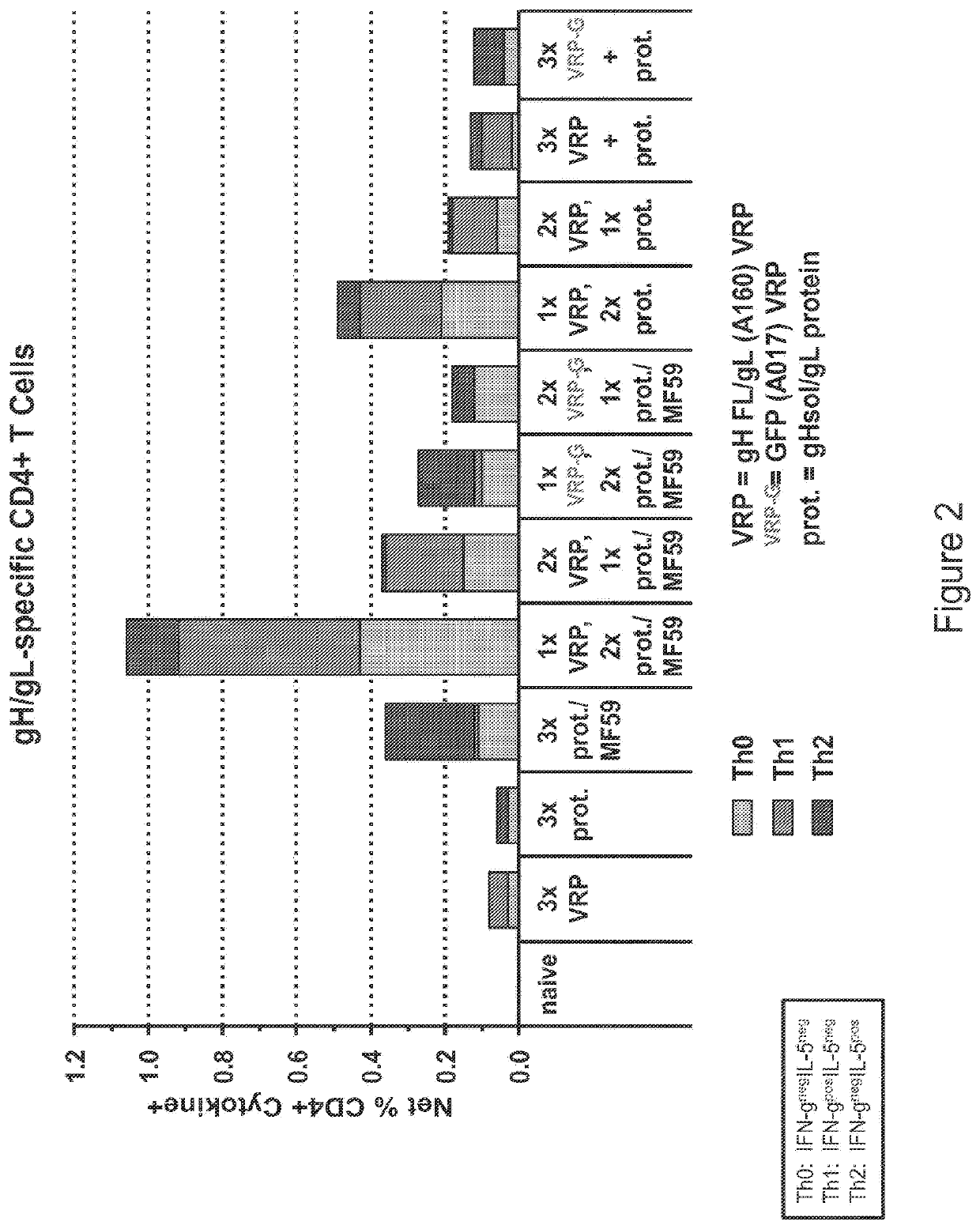
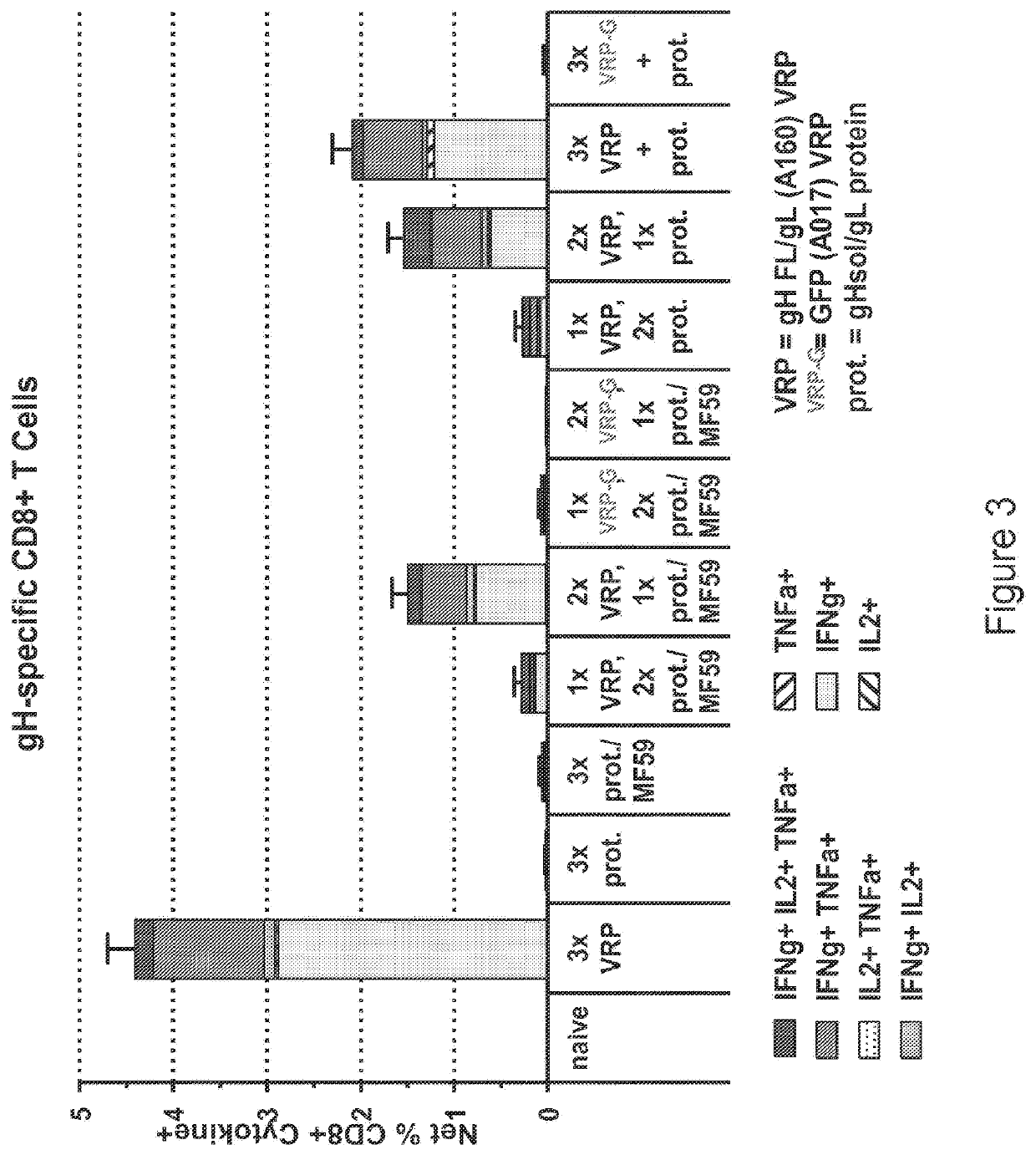
[0438]The vA142 replicon was used for this study. This construct expresses the full-length wild type surface fusion glycoprotein of RSV with the fusion peptide deleted and the 3′ end of the replicon is formed by ribozyme-mediated cleavage. BALB / c mice, 116 total, were divided into 11 groups (4-22 animals per group).
[0439]Group 1 (8 animals) were given bilateral intramuscular vaccinations (50 μL per leg) on days 0, 21 with unadjuvanted RSV-F subunit protein vaccine (3 μg) and all animals were sacrificed at day 42.
[0440]Group 2 (22 animals) were given bilateral intramuscular vaccinations (50 μL per leg) on days 0, 21 with the RSV-F subunit protein vaccine (3 μg) adjuvanted with alum. 4 animals were sacrificed at day 42. 6 mice were given a third vaccination at day 42 with the RSV-F subunit protein vaccine (3 μg) adjuvanted with alum and the animals were sacrificed at day 63. 6 mice were given a third va...
example iii
Administration of RNA and Protein to Rats (Study 1)
[0463]Three different replicons were used for this study: the vA317 replicon, which expresses the full-length wild type surface fusion glycoprotein of RSV (RSV-F); the vA318 replicon, which expresses the truncated (transmembrane and cytoplasmic tail removed) surface fusion glycoprotein of RSV; and the vA142 replicon, which expresses the full-length wild type surface fusion glycoprotein of RSV with the fusion peptide deleted. Cotton rats, 2-8 animals per group, were given intramuscular vaccinations (100 μL in one leg) on days 0 and 21 with the three different RNAs (vA317, vA318, vA142) formulated in LNPs (RV01(29) or CNE17 (N:P ratio 10:1) and given at two doses (1.0 and 0.1 μg, 8 animals / group). LNPs had the following composition: 40% DlinDMA, 10% DSPC, 48% Chol, 2% PEG DMG 2000, N:P ratio of 8:1 and was made using Method B, except a 150 μg RNA batch size was used. Control groups received the RSV-F subunit protein vaccine (5 μg) adj...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com