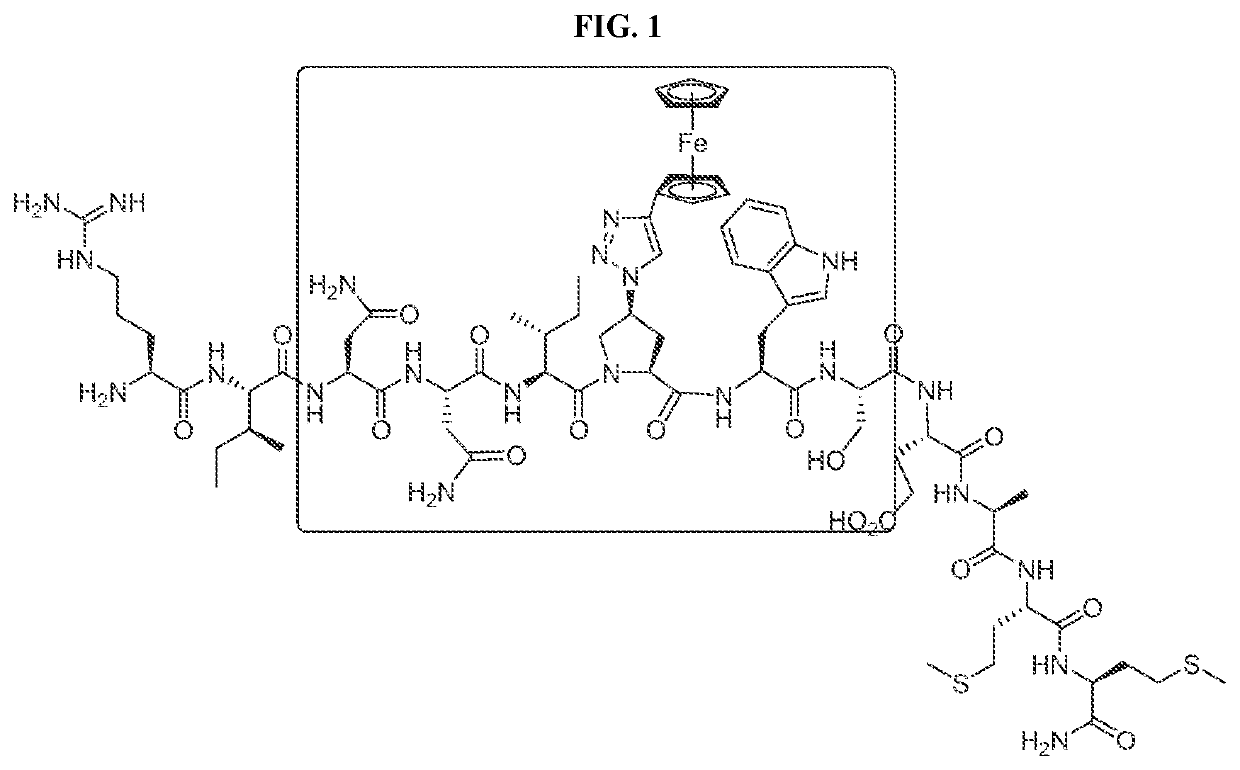
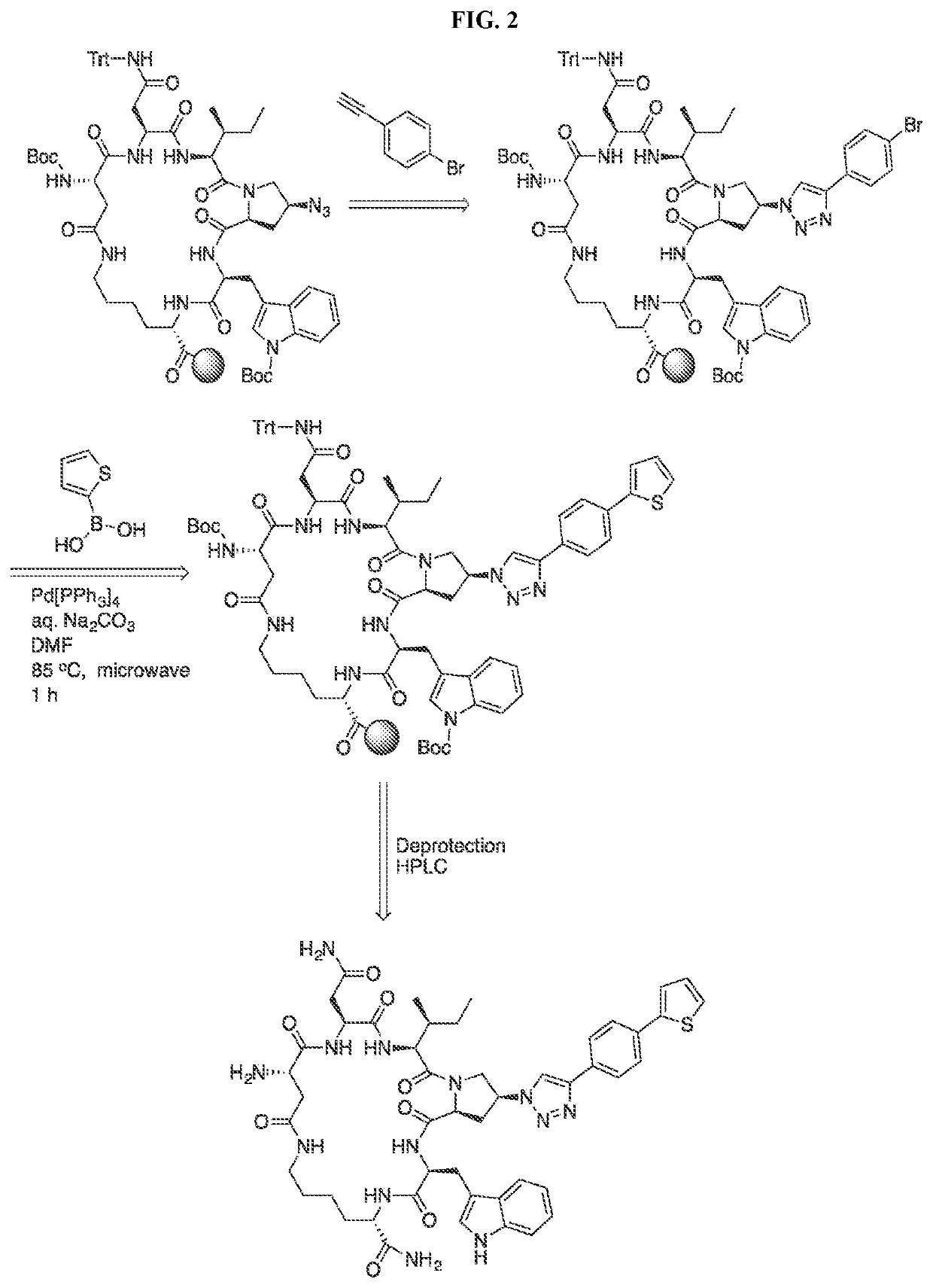
Cyclic Peptide Antiviral Agents and Methods Using Same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ptides
[0180]Cyclic peptides of the invention can be prepared using intramolecular cyclization according to the methods disclosed in International Application Publication No. WO 2016 / 094518, which is incorporated herein by reference in its entirety.
Chemical Synthesis of cPTs (3-38):
[0181]Alkynes a10-a16 were synthesized from the corresponding benzyl bromides a1-a9 following the procedures previously described (Louvel, et al., 2013, J. Med. Chem. 56:9427-40). Alkynes a10-a16 were used in click reactions without purification, because they are volatile and unstable upon storage. Mass validation of all cPTs was performed using Thermo Scientific LTQ XL Ion Trap LC / MS (Table 4).
General Synthesis of Alkynes a9-a16:
[0182]To a solution of ethynyltrimethylsilane (40 mmol) in 20 mL THF at 0° C., was added i-PrMgCl (2 M solution in THF) dropwise, and the mixture was stirred at 0° C. for 30 min, and allowed to warm up to r.t. for 30 min. CuBr (6 mmol) was added, and the mixture was stirred at r.t...
example 3
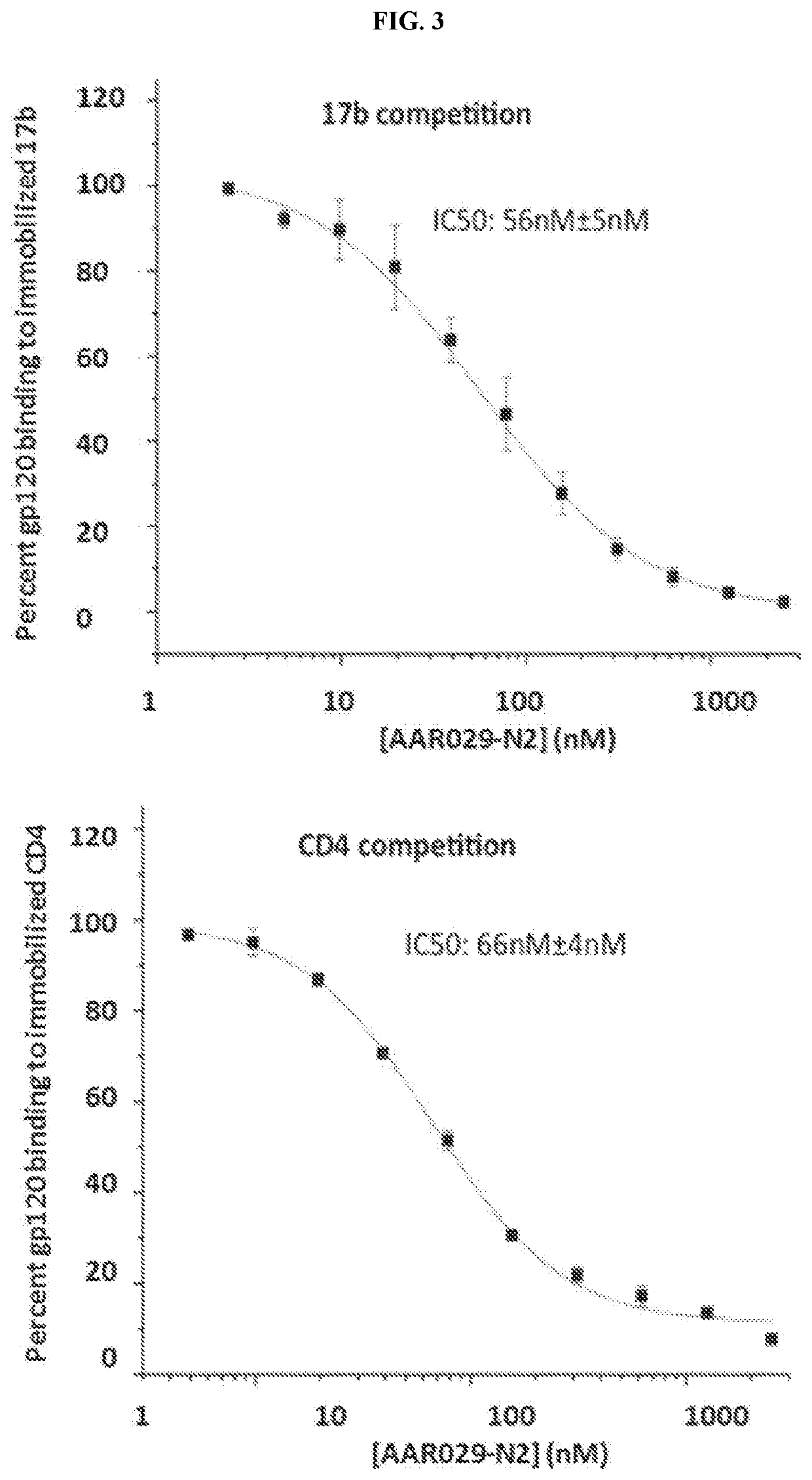
n of HIV-1 gp120 and Soluble CD4 Proteins
Reagents:
[0187]Escherichia coli strain Stbl2 cells were products of Novagen Inc. (Madison, Wis.). DNA plasmids encoding BaL.01 gp160 and NL4-3R-E-Luc+ were obtained from the NIH AIDS Reagent Program, Division of AIDS, NIAID. pcDNA3.1 vector carrying CD4 was a gift from Navid Madani. 17b IgG was purchased from Strategic Diagnostics Inc. (Newark, Del.). All other reagents used were of the highest analytical grade available.
Expression and Purification of Wild-Type gp120YU-2:
[0188]The DNA for gp120YU-2 in pcDNA3.1 vector for transient transfection was purified using a Qiagen MaxiPrep kit (Qiagen) after transforming into Stbl2 competent cells. The purified DNA encoding gp120 YU-2 was transfected into HEK 293F cells according to manufacturer's protocol (Invitrogen). Five days after transfection was initiated, cells were harvested and spun down (3000 RPM), and the supernatant was filtered through 0.2 m filters. Purification was performed over a 17b ...
example 4
n of HIV-1 gp120 Proteins
Surface Plasmon Resonance (SPR) Assays:
[0190]SPR experiments are performed on a Biacore 3000 optical biosensor (GE Healthcare). All experiments are carried out at 25° C. using standard PBS buffer pH=7.4 with 0.005% surfactant Tween and 2% DMSO.
[0191]Three flow cells in the CM5 chip were used for amine coupling of different ligands through standard 1-ethyl-3-(3-(dimethylamino)propyl) carbodiimide (EDC) / N-hydroxysuccinamide (NHS) chemistry. Flow cell 1 containing 2000 RUs of immobilized antibody 2B6R (α-human IL5R) served as a negative control for flow cells 2 and 3 each of which contained 2000 RUs of immobilized CD4 and 17b respectively.
[0192]For kinetic analyses, typically 2000-3000 RUs of protein reagents are immobilized on SPR chips, and analytes are passed over the surface at 50-100 μL / min. Surface regeneration is achieved by a 5 μL injection of 10 mM HCl solution at 100 μL / min. Analysis of peptide-mediated inhibition of gp120 binding to sCD4 and mAb 17b ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com