Methods and Compositions for Treating Pruritus, Xerosis, and Associated Disease Using CCR-Inhibitors
a technology of ccr3 and inhibitors, applied in the field of skin disorders, can solve the problems of increasing the burden on patients and caregivers, poor tolerance of oral corticosteroids at high doses, and poor drug tolerance, and achieves limited efficacy, drug tolerance, and unwanted side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
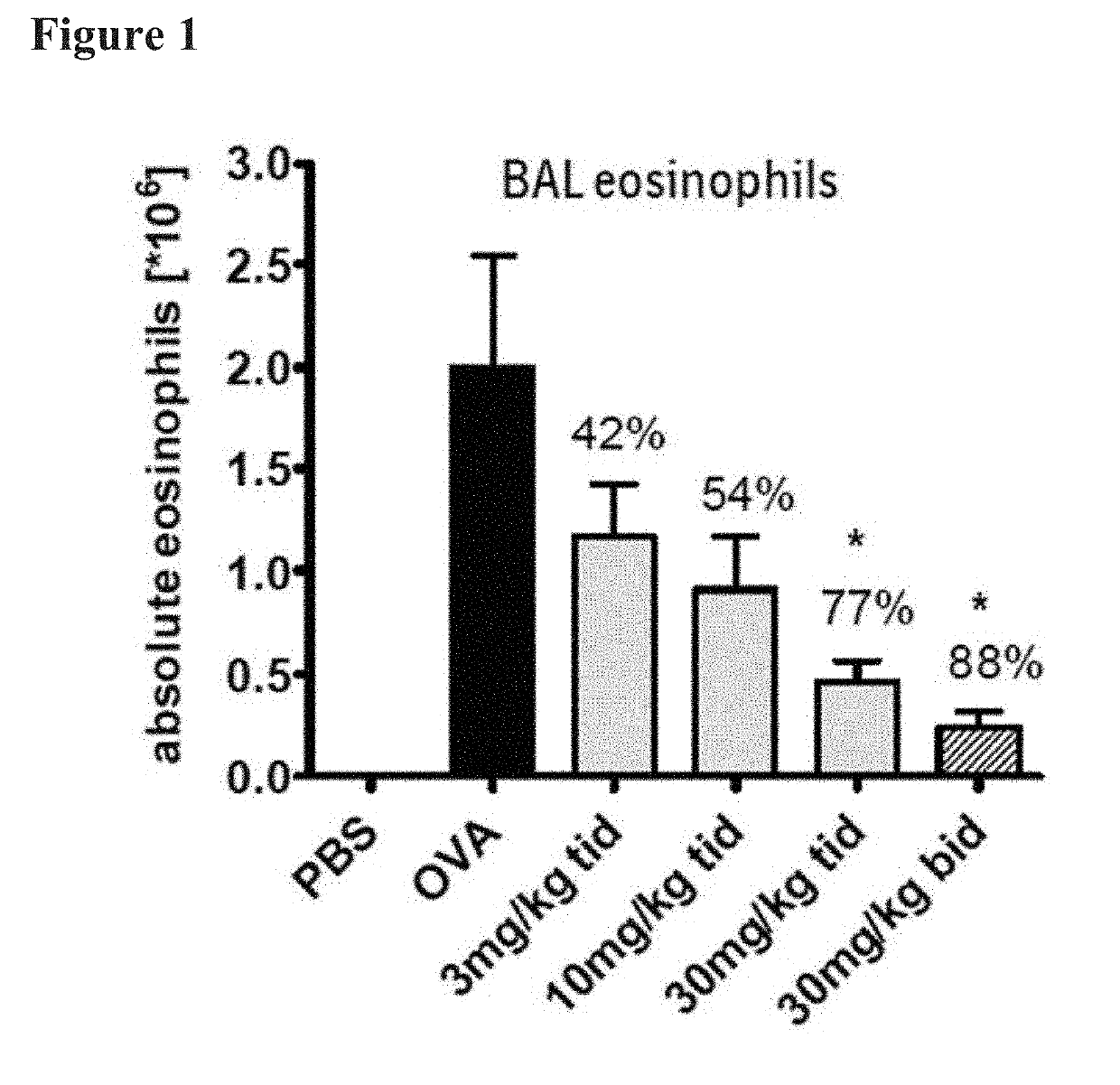
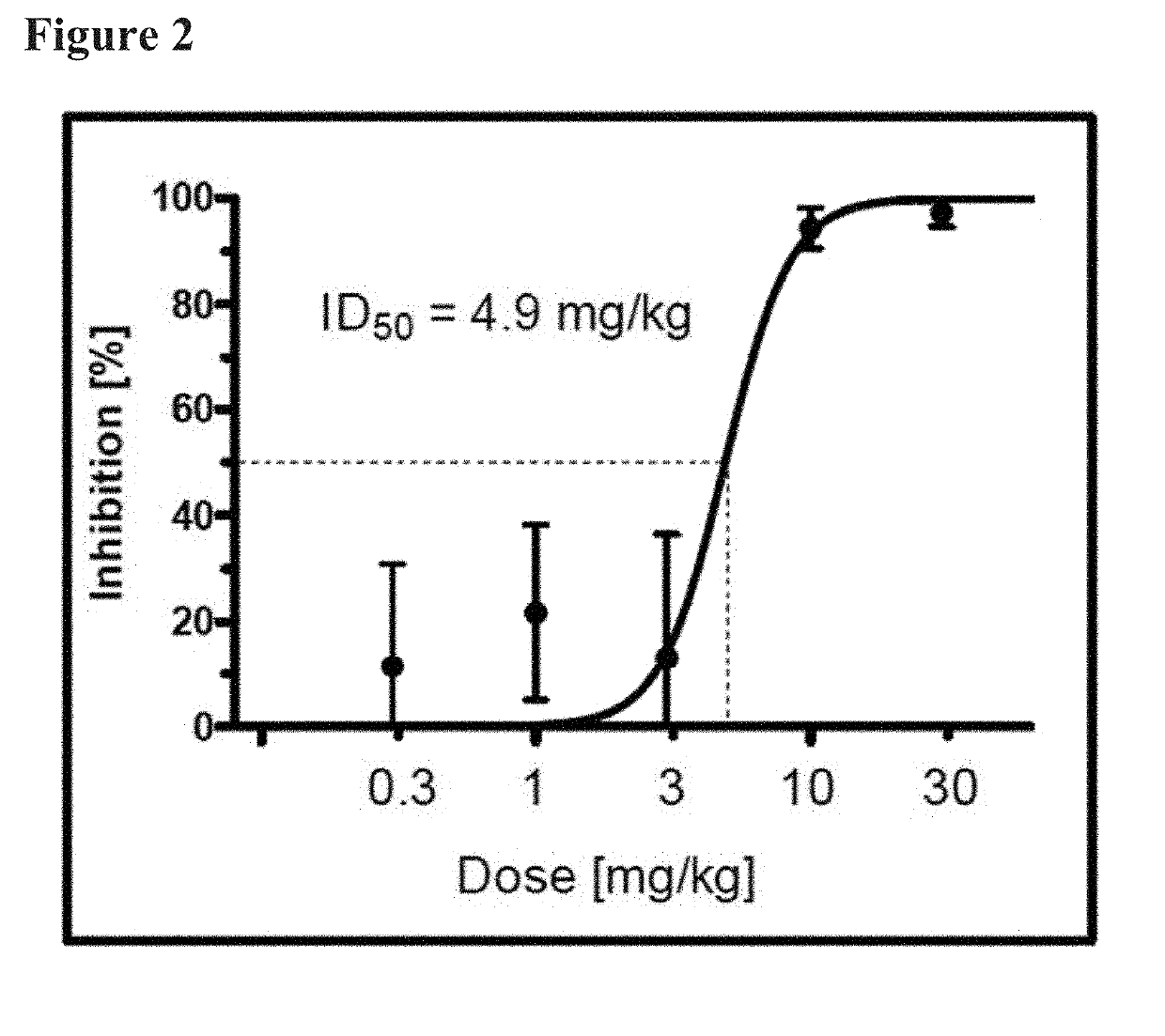
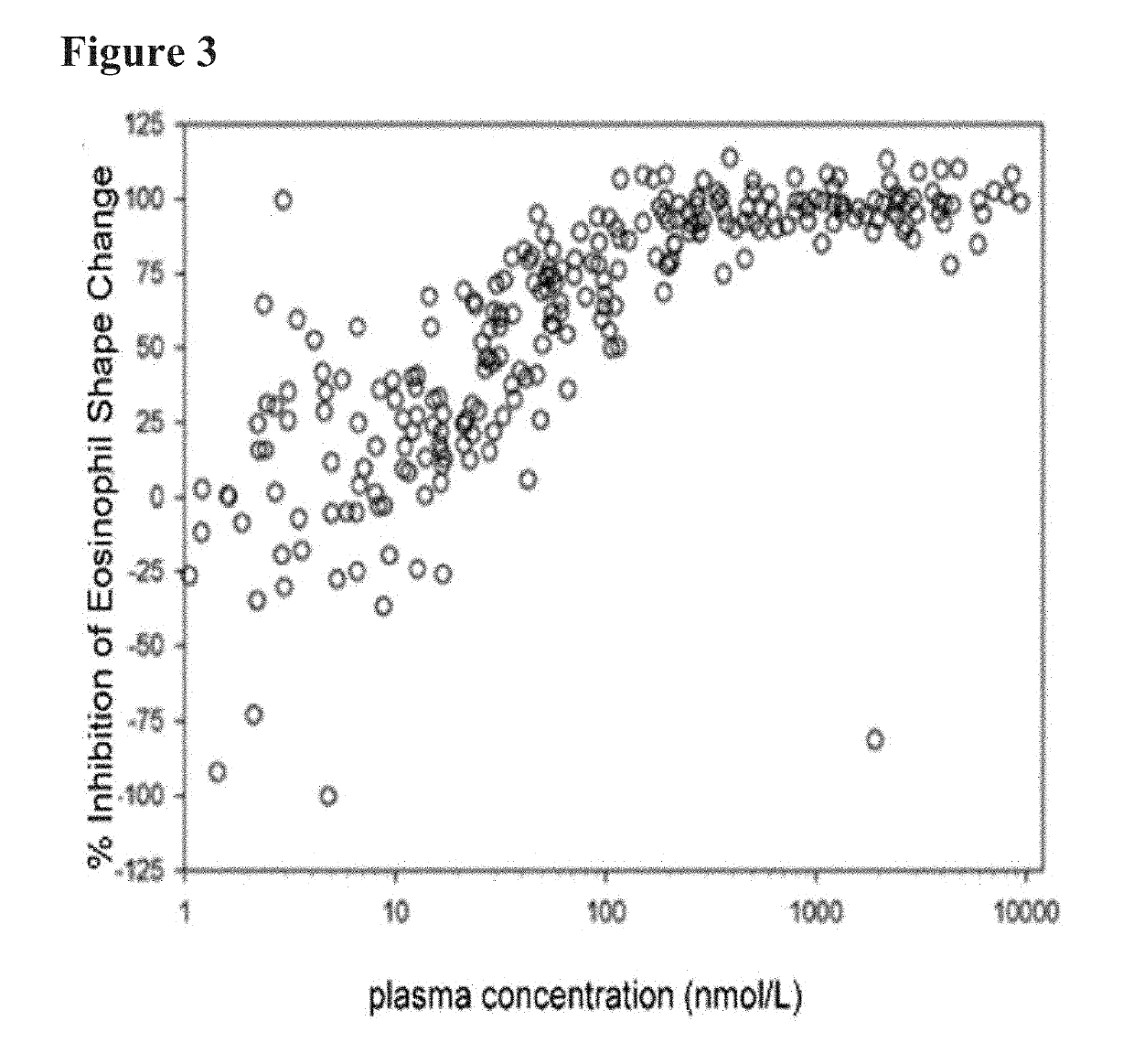
[0026]Aspects of the invention include methods of treating skin-disorders and corresponding symptoms such as pruritis and xerosis. The skin-disorders and corresponding symptoms may manifest themselves as pruritis and xerosis associated with dermatologic diseases including by way of example and not limitation, xerosis, dermatitis, dyshydrotic dermatitis, drug reactions, urticaria, atopic dermatitis / neurodermatitis, seborrheic dermatitis, psoriasis, palmoplantar pustulosis, lichen planus, pityriasis rubra pilaris, darier disease, Hailey-Hailey disease, Grover's disease, polymorphic light eruptions, bullous pemphigoid, acquired epidermolysis bullosa, dermatitis herpetiformis, pemphigus vulgaris, dermatomyositis, systemic sclerosis, Sjögren syndrome, Herpes simplex, Herpes zoster, tineas, candidal intertrigo; malassezia folliculitis, Ofuji's disease, scabies, lice, cutaneous larva migrans, insect bites / arthropod reactions, rosacea, mastocytosis, cutaneous lymphomas, mycosis fungoides, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| 2θ | aaaaa | aaaaa |
| 2θ | aaaaa | aaaaa |
| 2θ | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com