Analogues of hepcidin mimetics with improved in vivo half lives
a technology of mimetics and hepcidin, which is applied in the field of analogues of hepcidin, can solve the problems of excessive absorption of iron from the diet, loss of iron-regulatory function, and development of iron overload, and achieves the effects of increasing serum half-life, enhancing solubility, and increasing molecular weigh
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Peptide Analogues
[0301]Unless otherwise specified, reagents and solvents employed in the following were available commercially in standard laboratory reagent or analytical grade, and were used without further purification.
[0302]Procedure for Solid-Phase Synthesis of Peptides
[0303]Peptide analogues of the invention were chemically synthesized using optimized 9-fluorenylmethoxy carbonyl (Fmoc) solid phase peptide synthesis protocols. For C-terminal amides, rink-amide resin was used, although wang and trityl resins were also used to produce C-terminal acids. The side chain protecting groups were as follows: Glu, Thr and Tyr: 0-tButyl; Trp and Lys: t-Boc (t-butyloxycarbonyl); Arg: N-gamma-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl; His, Gln, Asn, Cys: Trityl. For selective disulfide bridge formation, Acm (acetamidomethyl) was also used as a Cys protecting group. For coupling, a four to ten-fold excess of a solution containing Fmoc amino acid, HBTU and DIPEA (1:1:1.1)...
example 2
Activity of Peptide Analogues
[0329]Peptide analogues were tested in vitro for induction of internalization of the human ferroportin protein. Following internalization, the peptides are degraded. The assay measures a decrease in fluorescence of the receptor.
[0330]The cDNA encoding the human ferroportin (SLC40 A1) was cloned from a cDNA clone from Origene (NM_014585). The DNA encoding the ferroportin was amplified by PCR using primers also encoding terminal restriction sites for subcloning, but without the termination codon. The ferroportin receptor was subcloned into a mammalian GFP expression vector containing a neomycin (G418) resistance marker in such that the reading frame of the ferroportin was fused in frame with the GFP protein. The fidelity of the DNA encoding the protein was confirmed by DNA sequencing. HEK293 cells were used for transfection of the ferroportin-GFP receptor expression plasmid. The cells were grown according to standard protocol in growth medium and transfect...
example 3
In Vivo Validation of Peptide Analogues
[0334]Hepcidin analogues of the present invention were tested for in vivo activity, to determine their ability to decrease free Fe2+ in serum.
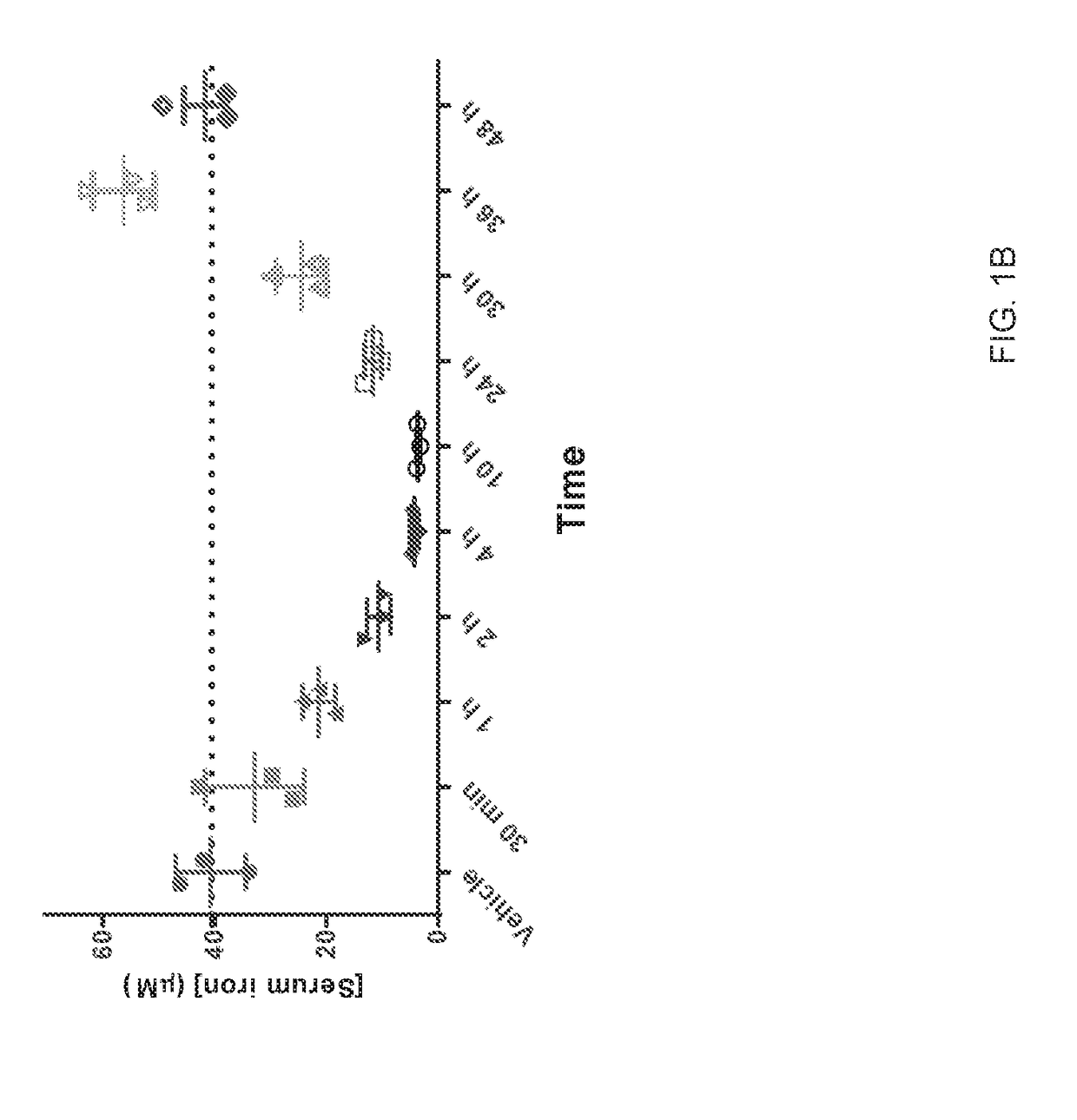
[0335]A hepcidin analogue (Compound 1) or vehicle control were administered to mice (n=3 / group) at 1000 nmol / kg either intravenously or subcutaneously. Serum samples were taken from groups of mice administered with the hepcidin analog at 30 min, 1 h, 2 h, 4 h, 10 h, 24 h, 30 h, 36 h, and 48 h post-administration. Iron content in plasma / serum was measured using a colorimetric assay on the Cobas c 111 according to instructions from the manufacturer of the assay (assay: IRON2: ACN 661). The data obtained from the cobas Iron2 analysis is presented in FIG. 1A (intravenous administration) and FIG. 1B (subcutaneous administration) as mean values+ / −SEM.
[0336]In another experiment, various hepcidin analogues or vehicle control were administered to mice (n=3 / group) at 1000 nmol / kg subcutaneously. Serum samples were...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com