Protein sequencing methods and reagents
a technology of protein and reagent, applied in the field of protein sequencing, can solve the problems of inapplicability to heterogeneous samples, low sensitivity of technology, and serious constraints of existing techniques, and achieve the effect of facilitating the chemical cleavage of the n-terminal amino acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
lecule Spectroscopy-Based Amino Acid Residue Identification
[0072]The inventor has adapted super-resolution fluorescence microscopy to an Edman-like sequencing process, allowing for the simultaneous, massively parallel identification and counting of large numbers of individual protein molecules. The protein molecules may be in vitro or in situ such as in cells and / or tissues. N-terminal amino acid residues of affixed polypeptides are reacted with a fluorescent probe that confers distinct spectral properties upon coupling to different amino acids. The resulting characteristic emission profile generated by the distinct N-terminal derivative formed on individual protein molecules is monitored by super-resolution spectroscopy to determine the identity of the corresponding cognate amino acid residue, which is then cleaved off, such as through Edman-like chemistry. Through multiple, iterative cyclic rounds of coupling the remaining polypeptide portions to fresh dye, re-imaging the populati...
example 2
ation and Characterization of Fluorescent Probes
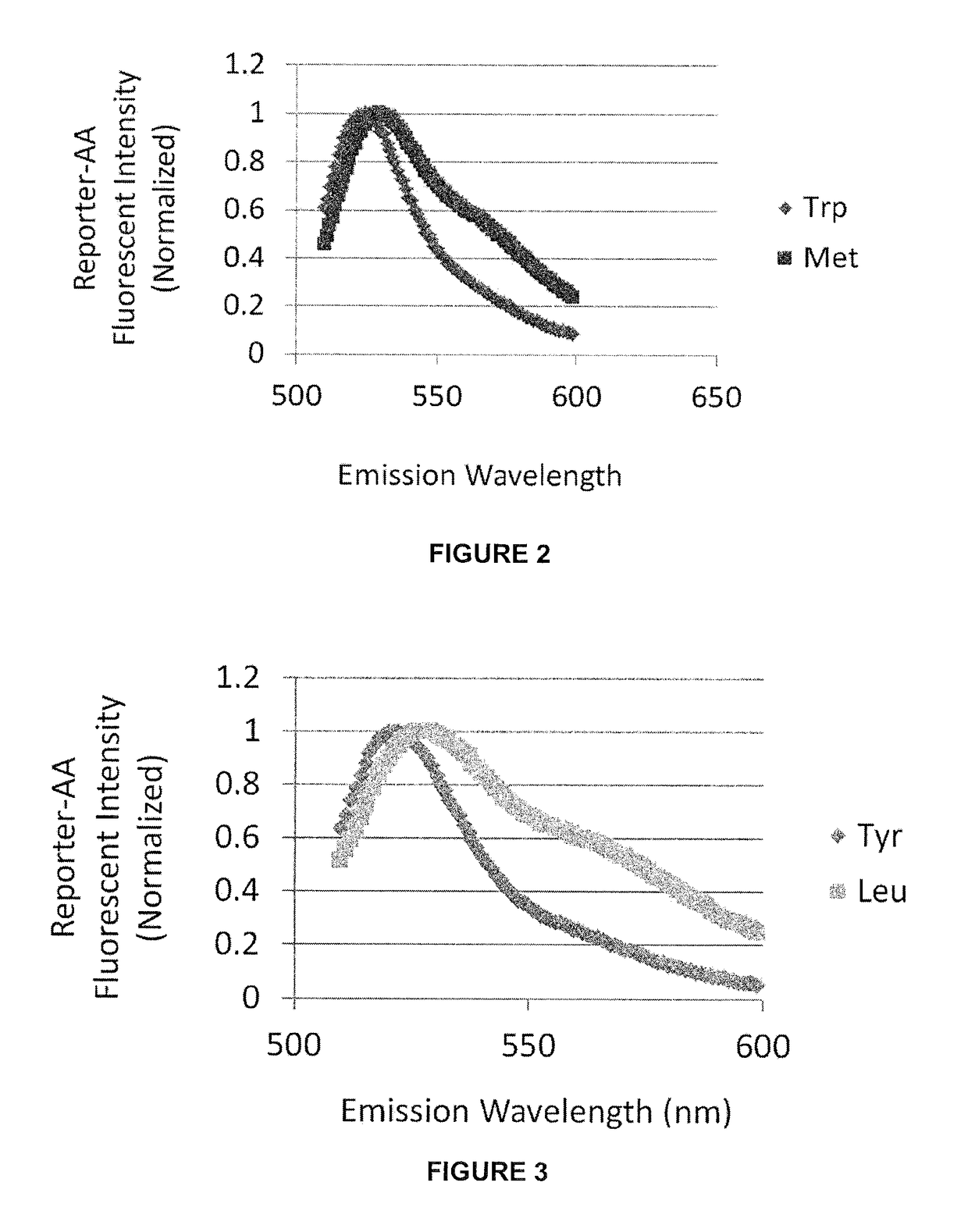
[0080]Using fluorescent probes some, or all, of the 20+ naturally occurring amino acids may be distinguished by the detecting the fluorescent emission of the individual probes conjugated to different amino acids. Various dye molecules, such HMRG and other xanthene-derivatives, are investigated for their suitability as probes for single molecule spectroscopy-based residue identification.
[0081]Experiments are conducted in order to identify fluorescent probes suitable for distinguishing different amino acids and to define optimal imaging conditions (eg. buffer pH / polarity) that maximize discrimination, as well as the influence, if any, of adjacent amino acids (eg. penultimate residue). Large numbers of individual probe molecules are examined after immobilization to a surface (eg. coverslip, flowcell, or microbead), or natively in / on metazoan cells, microbes or virus, in order to derive a precise, imaging-based N-terminal readout for one, ...
example 3
ITC Coupling and Identifying Different N-Terminal Amino Acid by Fluorescence Profiling
Materials and Methods
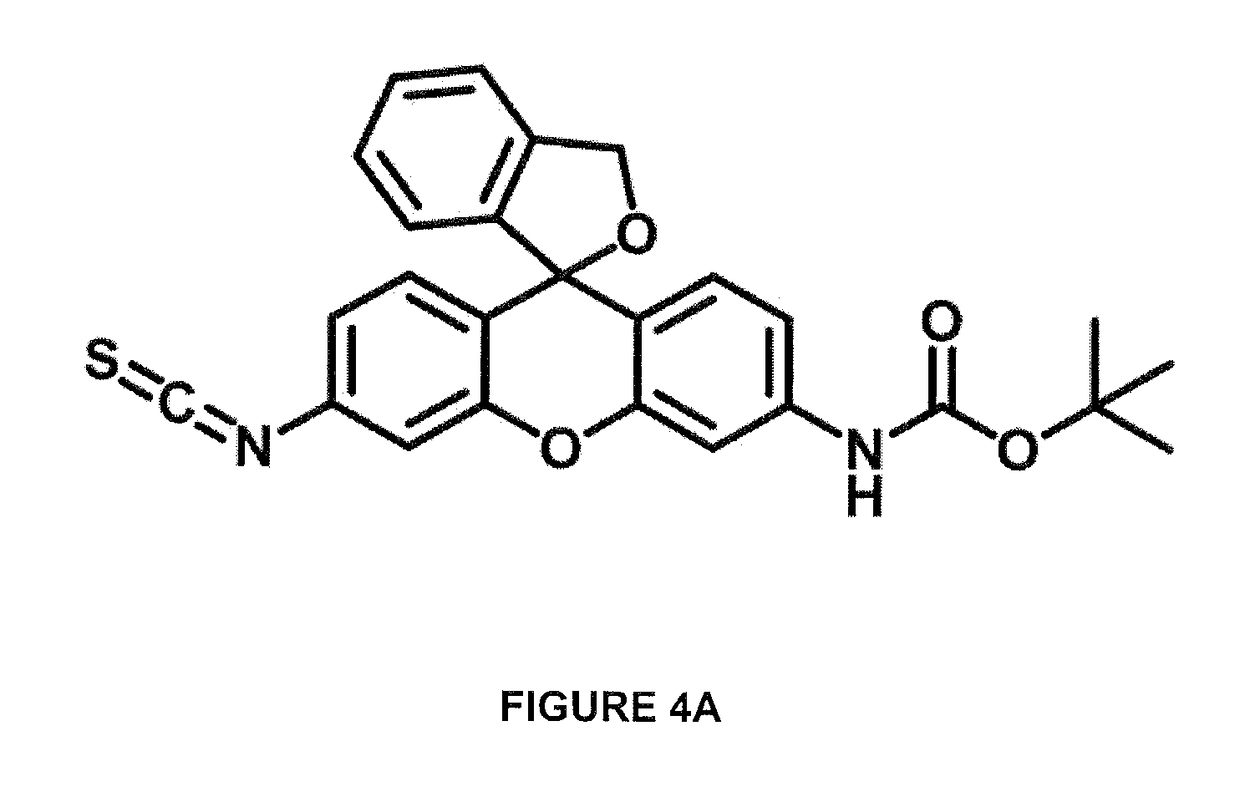
[0084]Hydroxymethyl rhodamine green (HMRG) tert-butyloxycarbonyl (BOC) isothiocyanate (ITC) (HMRG-BOC-ITC) as shown in FIG. 4A was synthesized as shown in FIG. 4B and dissolved in DMSO to a final concentration 34 nmol / μl.
[0085]Peptide beads: Peptide beads were synthesized by Kinexus (Vancouver B.C). Tentagel resin (Tenta-Gel® M NH2, 10 μm beads, RAPP Polymer) was used as the support for solid peptide synthesis. The peptides were synthesized to have the amino acid sequence X-AGWYMRLG (SEQ ID NO: 1), where X represents any one of 20 different amino acids at N-termini of the peptide. Approximately half (˜50%) of each peptide / resin contained a cleavable HMBA linker inserted between peptide sequence and the bead which enabled cleavage of the peptide molecules from the beads upon treatment with base.
[0086]Prior to coupling, 100 μl of dimethylformamide (DMF) was added to each tube con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
| slit width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com