Ferrite as three-way catalyst for treatment of exhaust gas from vehicle engine
a technology of ferrite and exhaust gas, which is applied in the direction of machines/engines, mechanical equipment, separation processes, etc., can solve the problems of inability to meet environmental requirements, fuel component and engine combustion design already reach the limit, and can't meet the serious air pollution, etc., to achieve the effect of wide temperature range, optimal catalytic effect and effective acceleration of scr reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment one
u-Ferrite (Cu:Fe=1:2.5)
[0030]A method for preparing Cu-ferrite is provided in this embodiment.
[0031]Weight CuSO4 and FeSO4 accurately according to the molar ratio required (Cu2+:Fe2+=1:2.5). Place them into the reactor and add 1 L deionized water with stirring to dissolve CuSO4 and FeSO4 completely. Add 6N sodium hydroxide (NaOH) into the solution and adjust the pH of the solution to 9.5. Then heat the solution to 85° C. After the temperature and the pH value becoming stable, introduce air into the solution at 3 L / min volumetric flow rate. Maintain the reaction condition until oxidation reduction potential (ORP) meter readings turn and increase rapidly. A solid crude product, Cu-ferrite, is obtained.
embodiment two
n-Ferrite (Mn:Fe=1:2.5)
[0032]A method for preparing Mn-ferrite is provided in this embodiment.
[0033]Weight MnSO4, FeCl2 and FeCl3 accurately according to the molar ratio required (Mn2+:Fe2+:Fe3+=1:0.167:2.333). Place them into the reactor and add 1 L deionized water with stirring to dissolve CuSO4, FeCl2 and FeCl3 completely. Heat the solution to 80° C. in a water bath. Then introduce nitrogen gas into the solution for 5 minutes and add ammonium hydroxide into the solution to make the metals precipitate completely. Keep stirring and heating the solution for 2 hours to get a solid crude product, Mn-ferrite.
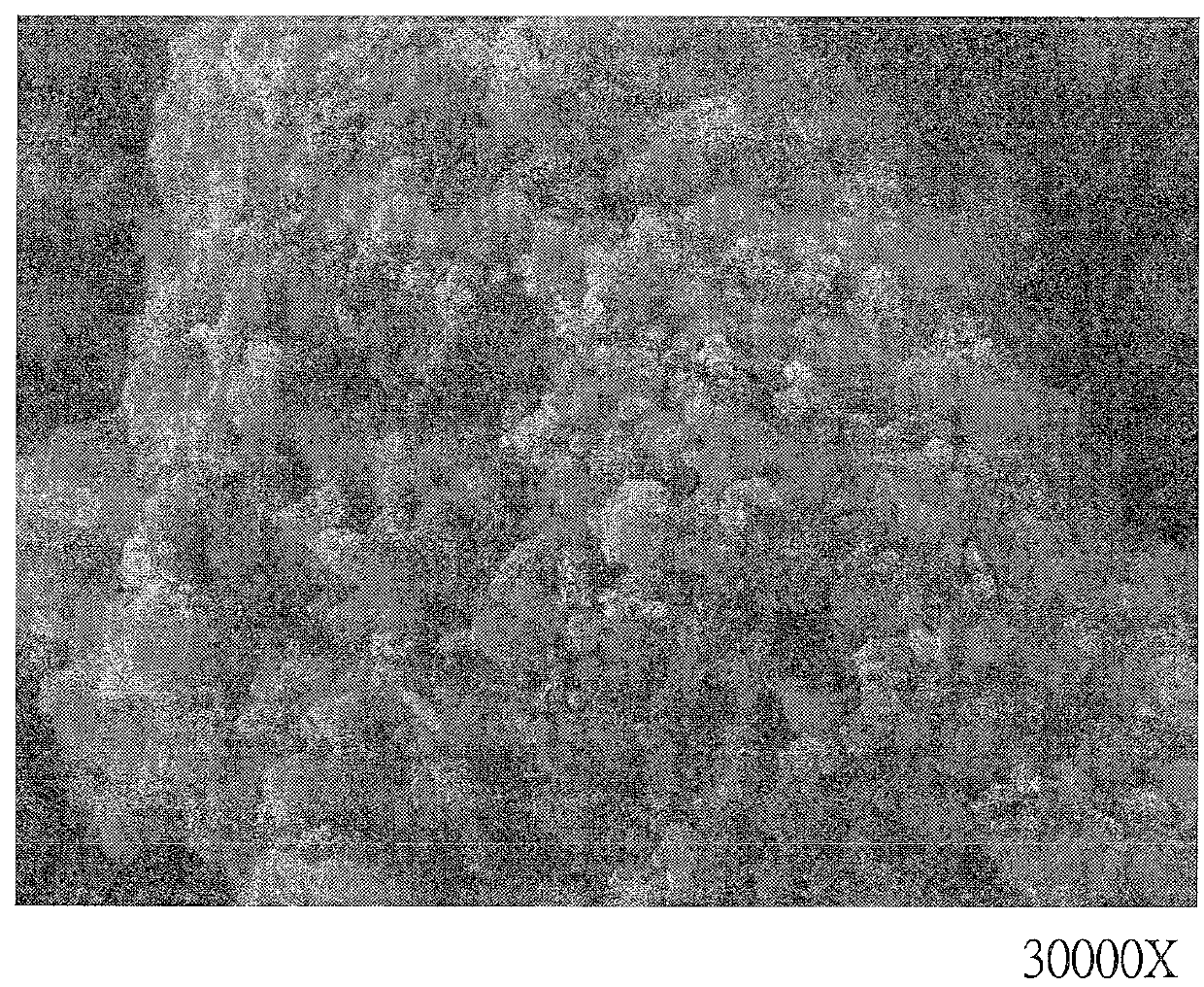
[0034]Refer to FIG. 1, the solid crude product is separated, dried, ground and sieved to get copper ferrite powder. The copper ferrite powder is observed by scanning electron microscope at 30000× magnification and each particle of the catalyst is formed by a plurality of nanoparticles with different sizes.
embodiment three
ficiency of Ferrite for Removal of Carbon Monoxide at Low Oxygen Concentration
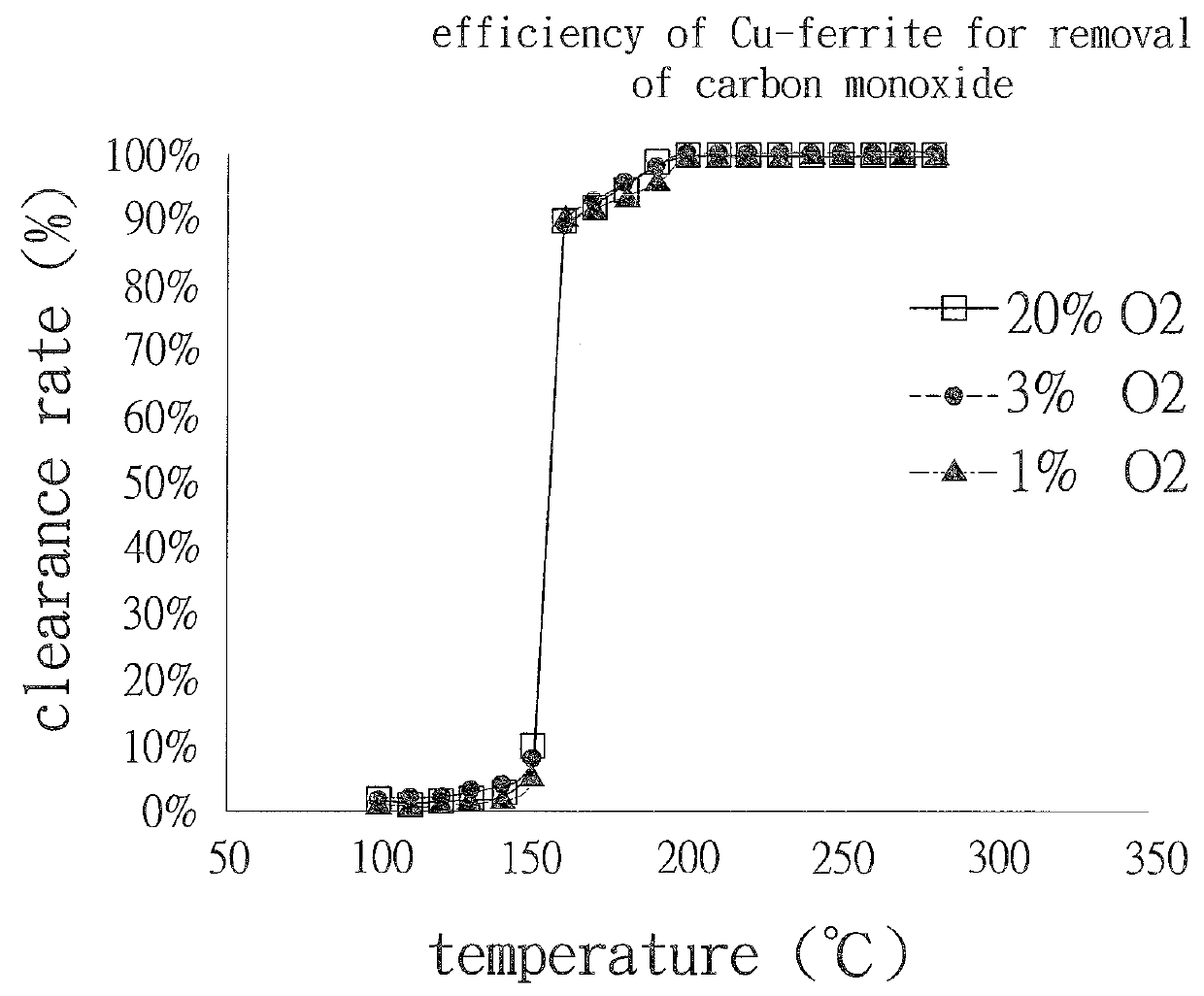
[0035]Refer to FIG. 2, Cu-ferrite catalyst can completely convert CO to CO2 for removal of CO at the temperature lower than 160° C. under the condition that the oxygen concentration is only 1%.
Embodiment Four: Discussion of Efficiency of Ferrite for Removal of Hydrocarbons at Low Oxygen Concentration
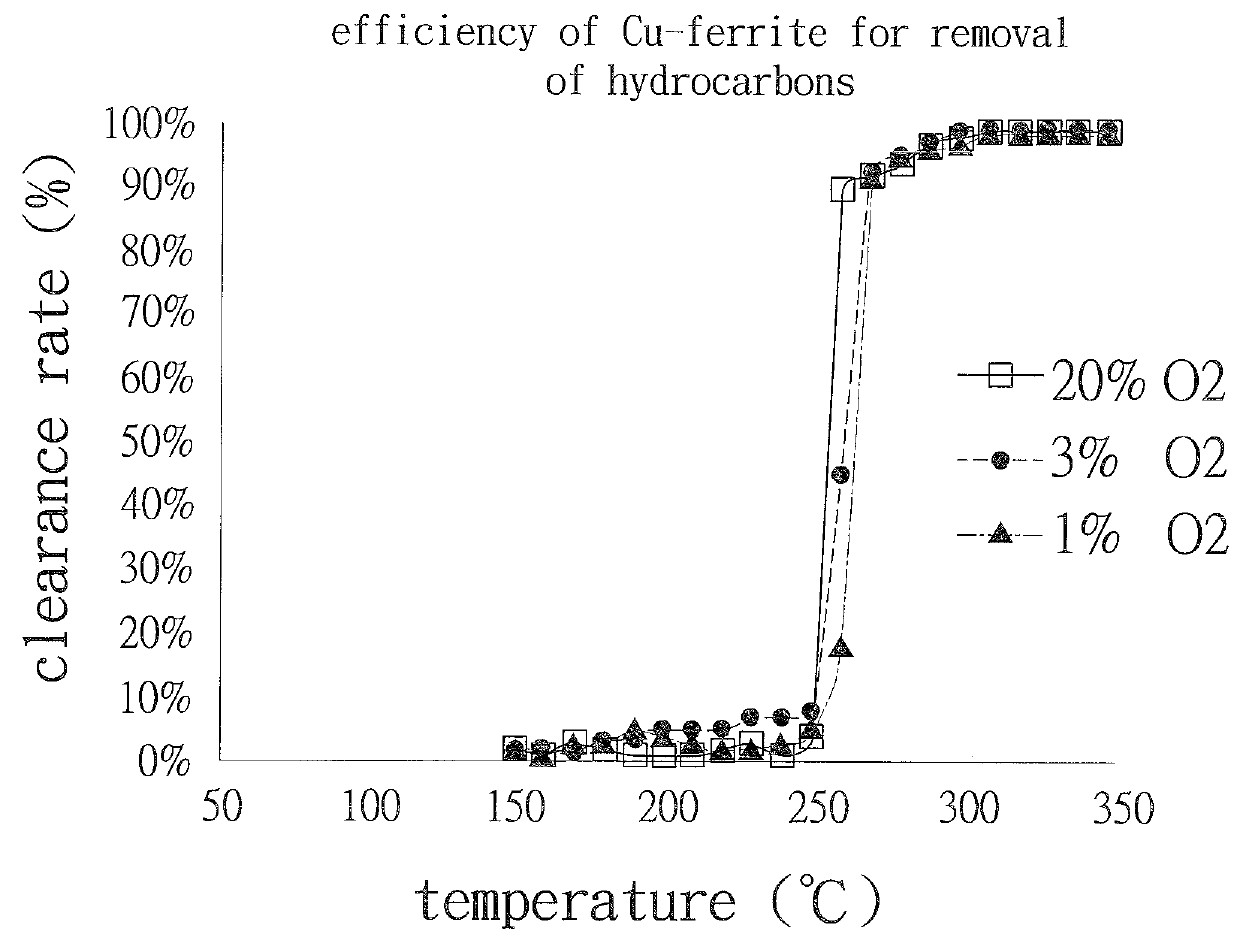
[0036]Refer to FIG. 3, Cu-ferrite catalyst can still remove hydrocarbons clearly at the temperature lower than 270 t under the condition that the oxygen concentration is only 1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com