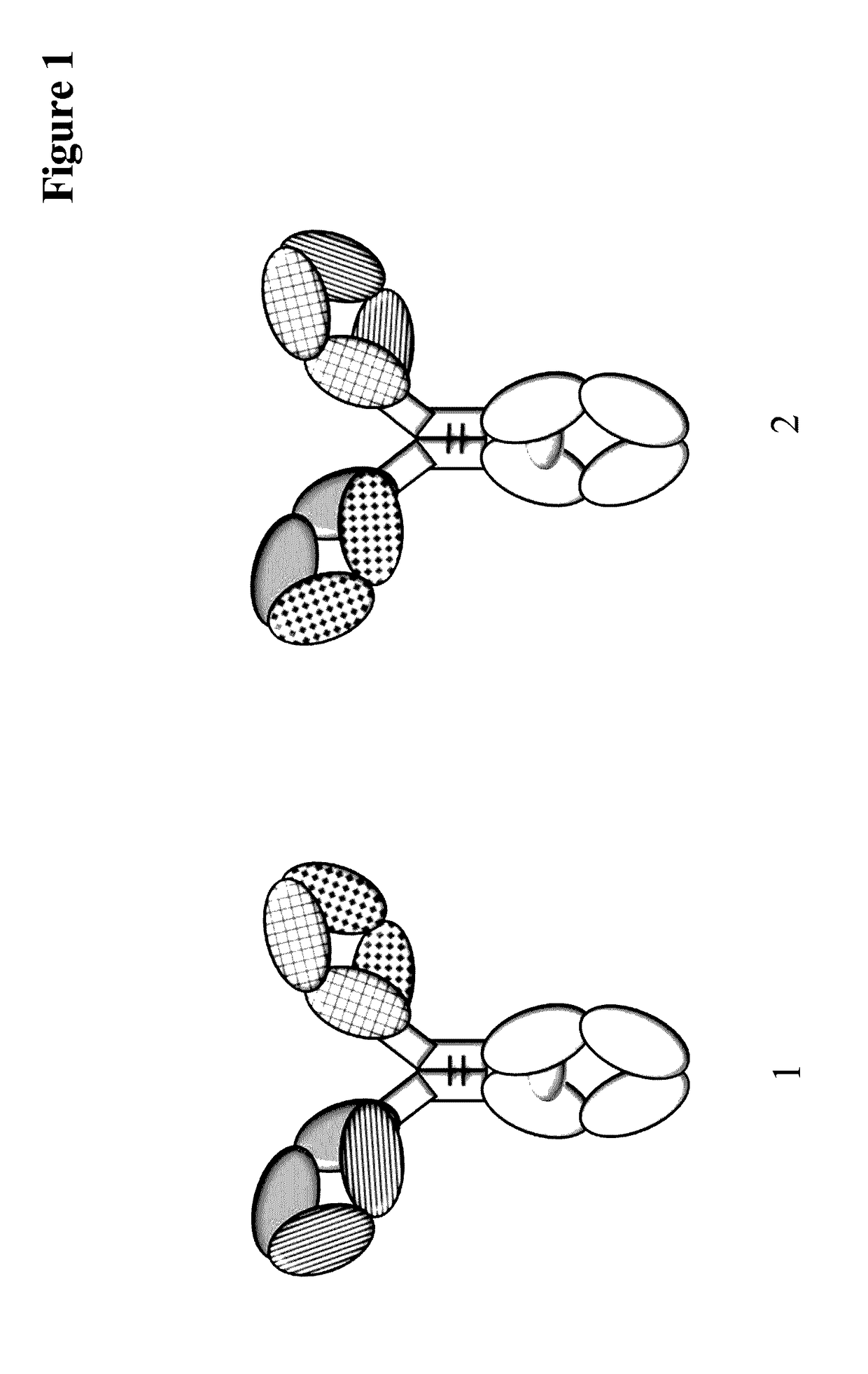
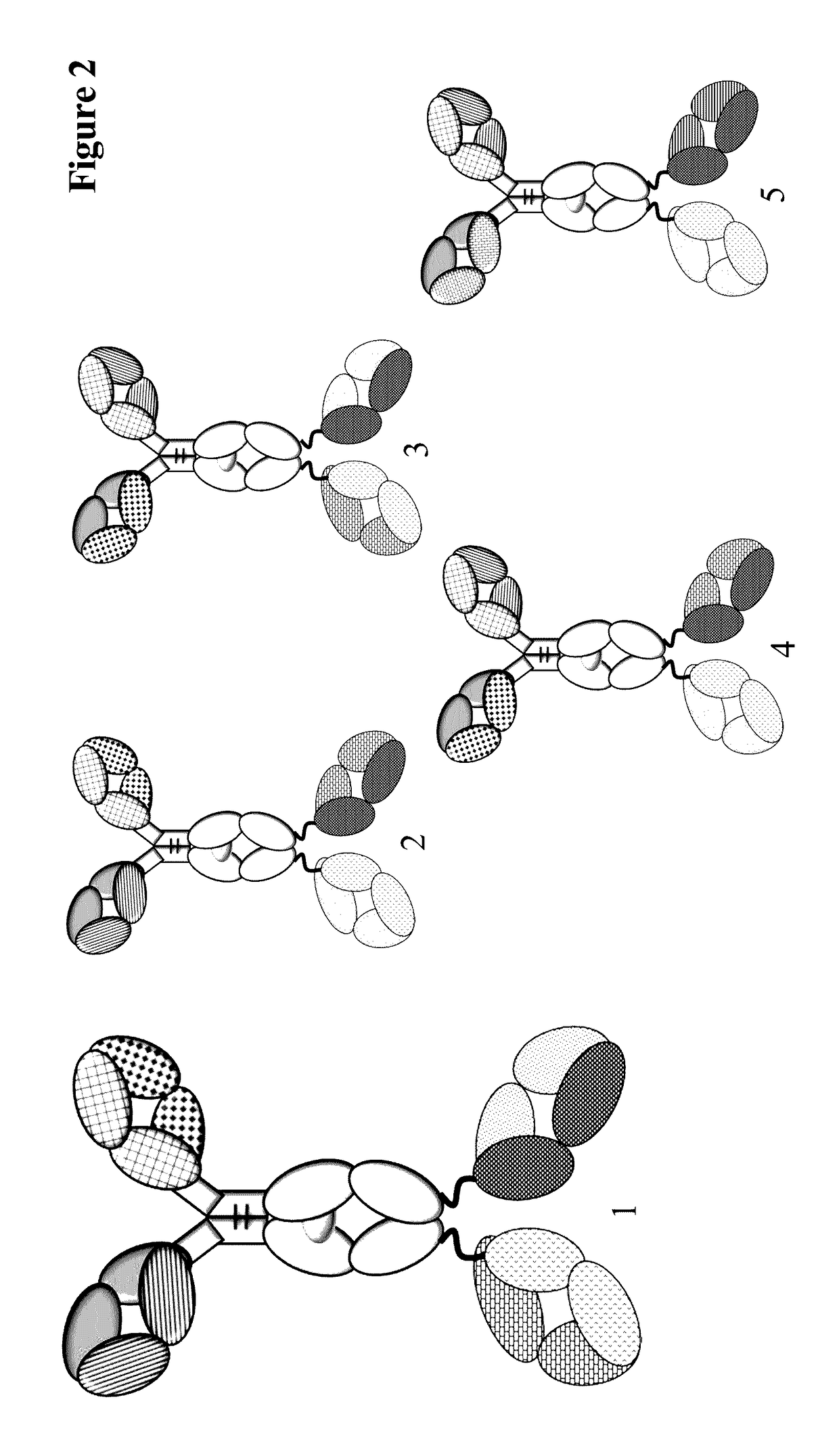
Method for detecting multispecific antibody light chain mispairing
a multi-specific antibody and light chain mispairing technology, applied in the field of multi-specific antibody light chain mispairing detection, can solve the problem of hampered light chain mispairing in a complete multi-specific antibody
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
specific embodiments
[0314]1. Use of a limited digestion with a proteolytic enzyme of a multispecific antibody for the analysis of the multispecific antibody's light chain pairing.[0315]2. Use of a limited digestion with a proteolytic enzyme of a multispecific antibody for the determination of light chain mispairing in the multispecific antibody.[0316]3. Use of a limited digestion with a proteolytic enzyme of a multispecific antibody produced by a recombinant mammalian cell for the selection of a multispecific antibody producing mammalian cell.[0317]4. The use according to any one of embodiments 1 to 3, wherein the proteolytic enzyme is selected from the group consisting of Lys-C, Asp-N, Arg-C, Glu-C and chymotrypsin.[0318]5. The use according to embodiment 4, wherein the proteolytic enzyme is Lys-C.[0319]6. The use according to any one of embodiments 1 to 5, wherein the incubating is for 35 to 45 minutes.[0320]7. The use according to embodiment 6, wherein the incubating is for about 40 minutes.[0321]8....
example 1
[0394]Method for the Determination of Light Chain Mispairing of a Multispecific Antibody after Limited Proteolytic Digestion
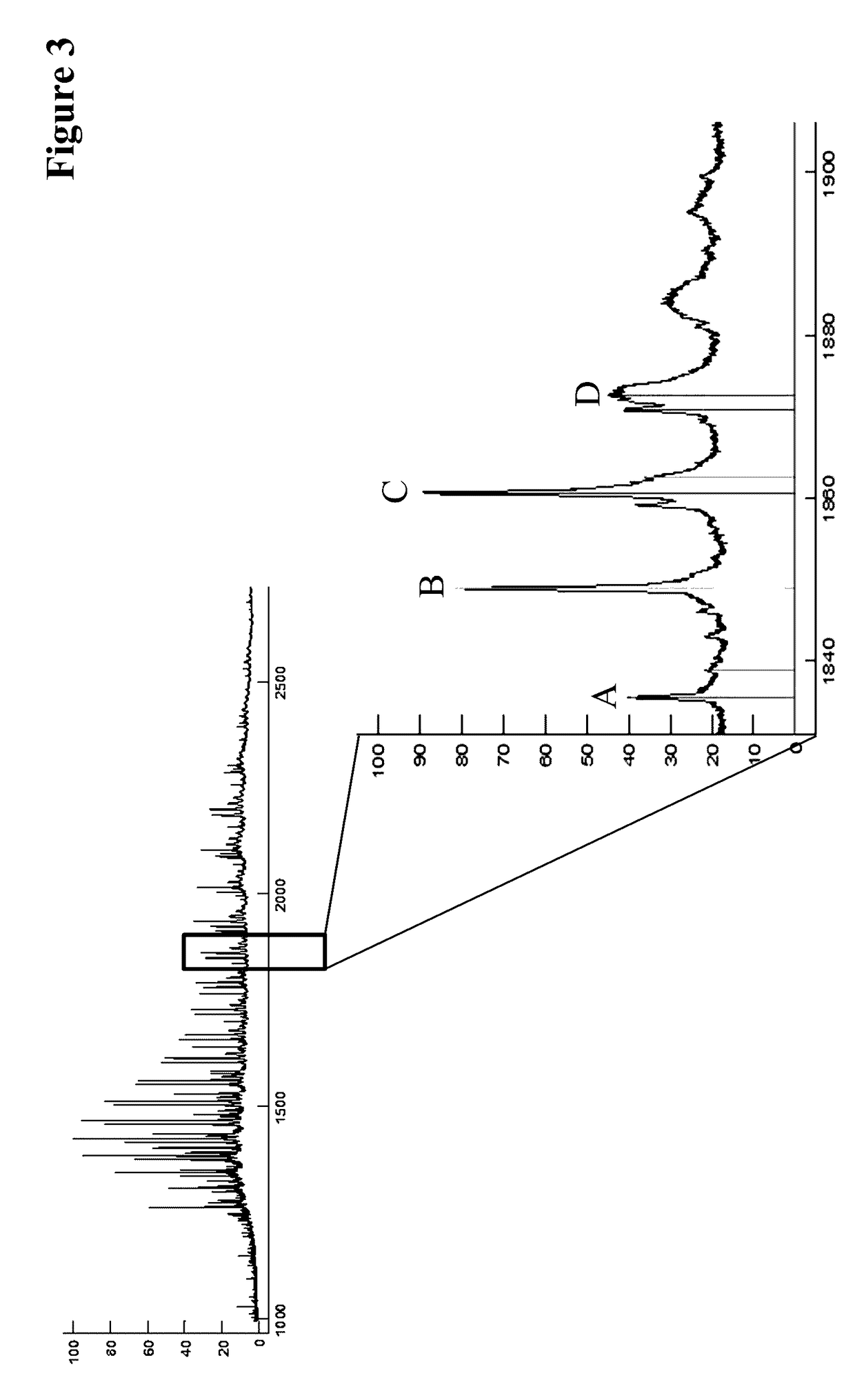
[0395]The expected primary structures were analyzed by electrospray ionization mass spectrometry (ESI-MS) of the limited LysC digested CrossMabs. Advantageously the antibody has been deglycosylated in advance.
[0396]The VH / VL CrossMabs was deglycosylated with N-Glycosidase F in a phosphate or Tris or histidine buffer at 37° C. for up to 17 h at a protein concentration of 1 mg / mL and an antibody: enzyme ratio of 100:1. The limited Lys-C (Roche Diagnostics GmbH, Mannheim, Germany) digestions was performed with 100 μg deglycosylated VH / VL CrossMabs in a Tris buffer pH 8 at 37° C. for 40 min.
[0397]Prior to mass spectrometry the samples were desalted via HPLC on a Sephadex G25 column (GE Healthcare).
[0398]The total mass was determined via ESI-MS on a maXis 4G UHR-QTOF MS system (Bruker Daltonik) equipped with a TriVersa NanoMate source (Advion).
example 2
Method for the Determination of Light Chain Mispairing of a Tetravalent Bispecific Antibody
[0399]The tetravalent bispecific antibody was deglycosylated with N-Glycosidase F in a phosphate or Tris or histidine buffer at 37° C. for up to 17 h at a protein concentration of 1 mg / ml and an antibody:enzyme ratio of 100:1. The limited Lys-C(Roche Diagnostics GmbH, Mannheim, Germany) digestions was performed with 100 μg deglycosylated tetravalent bispecific antibody in a 100 mM citrate buffer pH 3.7 at 37° C. for 16 hours at a protein concentration of approx. 0.9 mg / mL and an antibody:enzyme ratio of 50:1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| weight ratio | aaaaa | aaaaa |
| weight ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com