Method for inducing pluripotent stem cells and pluripotent stem cells prepared by said method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Preparation of Pluripotent Cells Such as Embryonic Stem Cells by Culturing Extract-Injected Cells
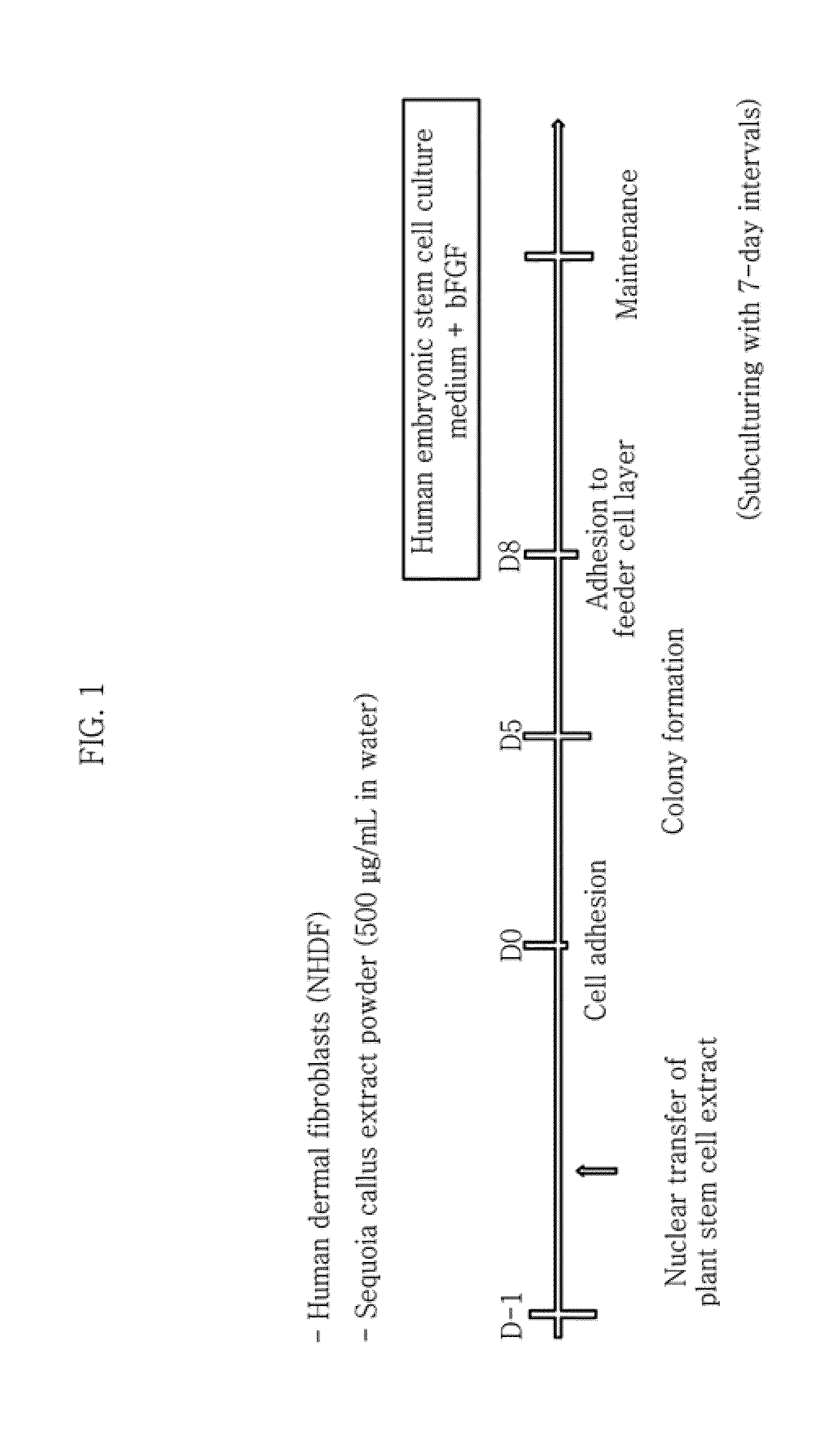
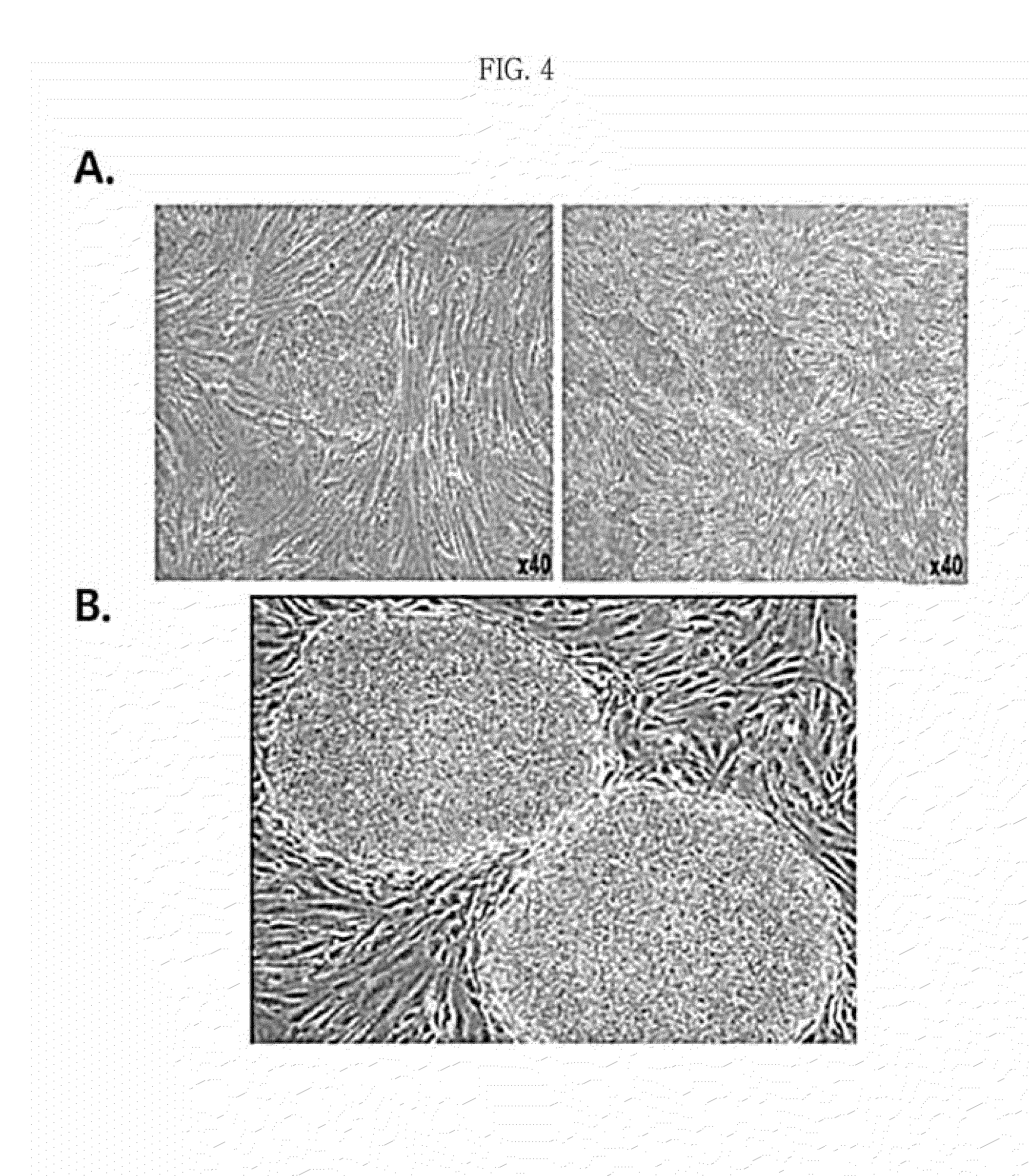
[0160]The extract-injected cells were incubated in a normal cell culture medium wherein DMEM (Dulbecco's modified Eagle's medium) was supplemented with 10% FBS, 50 U / mL penicillin and 50 mg / mL streptomycin in an incubator maintained at 37° C. and 5% CO2. The adult-derived cells (human-derived dermal fibroblasts) into which the plant stem cell extract (callus powder) was injected were cultured on a dish coated with 0.1% gelatin. The medium was replaced after the first two days. After culturing for 10 days while replacing the medium every day, the cells were transferred to a feeder cell (STO cell) layer treated with mitomycin C (MMC) at a ratio of 1:2. Then, the cells were transferred to a new feeder cell layer with 7-day intervals while replacing DMEM (Dulbecco's modified Eagle's medium) / F12 supplemented with 20% KSR (knockout serum replacement), 2 mM L-glutamine, 0.1 mM nonessential amin...
example 3
Characterization of Induced Pluripotent Stem Cells (Gene Expression Analysis)
[0163]The cultured cells were recovered and total RNA was separated by using the TRIzol reagent (Invitrogen). After synthesizing cDNA through reverse transcription polymerase chain reaction (RT-PCR), PCR was conducted using primers specific for the Nanog and Oct3 / 4 genes and the GAPDH gene as a control gene. The expression of these genes was analyzed by electrophoresing the PCR product on an agarose gel. The result is shown in FIG. 7.
[0164]As seen from FIG. 7, the pluripotent stem cells (hiPS) induced by the method of the present disclosure showed expression of the Nanog and Oct3 / 4 genes, which are characteristic of embryonic stem cells (hES).
example 4
Preparation of Sequoia (Sequoiadendron giganteum) Callus Extract Containing Shikimic Acid
[0165]20 mg of the sequoia callus powder of Example 1 was dissolved in 1 mL of a DMSO solvent. Similarly, 1 g of the sequoia callus powder was dissolved in 10 mL of a mixture solvent of BG and EtOH to prepare a sequoia callus extract containing shikimic acid. The prepared extracts were used as samples in the following test example.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com