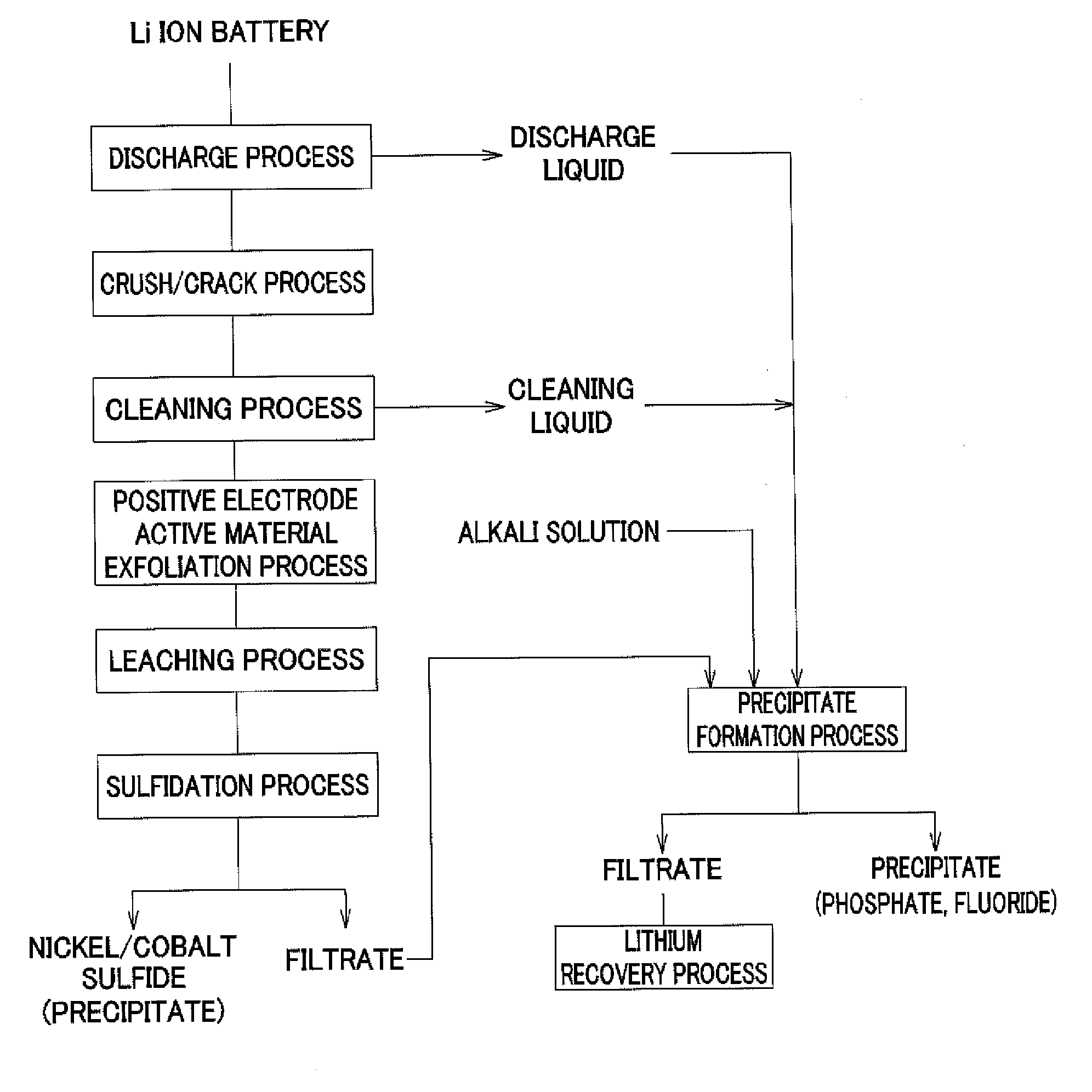
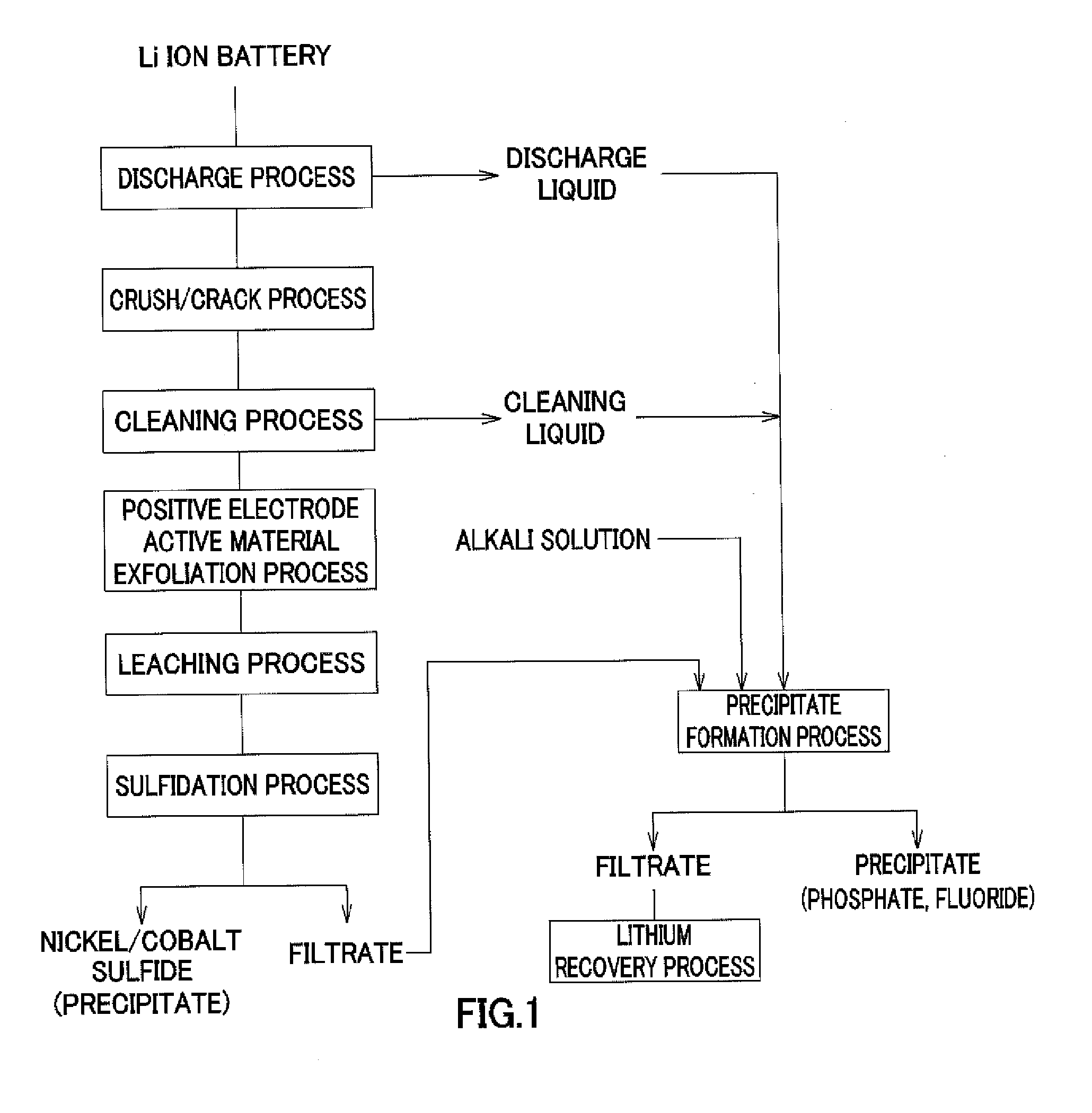
Method for recovering lithium
a lithium ion battery and lithium ion battery technology, applied in the field of lithium recovery from lithium ion batteries, can solve the problems of lithium being made into slag and becoming unrecoverable, difficult separation and refinement, and energy consumption and exhaust gas treatment, etc., and achieve the effect of efficient separation and removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0103]In the above-described operation of recovering valuable metals from a used lithium ion battery, by filtering of the slurry recovered after the discharge treatment and the cleaning treatment, and the slurry of post-sulfidation process, the process liquid made of the discharge liquid and the cleaning liquid of post-treatment and the filtrate of post-sulfidation process were obtained. Table 1 shows composition of the obtained solution.
TABLE 1LiPFComposition of lithium-containing solution (g / l)5.30.41.3
[0104]As shown in Table 1, the solution contained lithium based on lithium hexafluorophosphate of a positive electrode active material. The following operation was continued by using the lithium-containing solution as an object from which lithium is recovered.
[0105]First, an 8 mol / l sodium hydroxide (NaOH) solution was added to the lithium-containing solution of 100 ml shown in Table 1, the solution was adjusted to have pH 9.5, and hexafluorophosphate ions contained in the solution ...
example 2
[0111]In Example 2, an operation was performed by a similar method to Example 1 except that an 8 mol / l potassium hydroxide (KOH) solution was used as the alkali hydroxide to be added instead of the sodium hydroxide solution in the hydrolysis treatment of the hexafluorophosphate ions by addition of alkali hydroxide and in the precipitate formation treatment in Example 1. Table 5 shows analysis values of the filtrate of post-hydrolysis treatment with the potassium hydroxide solution and a precipitate formation treatment.
TABLE 5LiPFComposition of solution after addition of KOH3.70.050.12(g / l)
[0112]As shown in Table 5, when the potassium hydroxide solution was added, the solubility of a phosphate and a fluoride salt was able to be decreased, compared with the case where the sodium hydroxide solution was added in Example 1, and a precipitate of the slightly soluble salts was able to be efficiently removed. Therefore, the content of phosphorus and fluorine in the filtrate was able to be f...
example 3
[0113]In Example 3, after a solution made of the composition of Table 2 was obtained by the same method as Example 1, next, a sodium carbonate solution or a potassium carbonate solution was added until saturated, and lithium was recovered as a crystal of lithium carbonate by carbonation treatment. That is, different from Example 1, filtrate after precipitate treatment was subjected to the carbonation treatment instead of the solvent extraction treatment, and lithium was recovered. Table 6 shows analysis values of a mother liquor of post-carbonation treatment, and Table 7 shows the quality of a crystal of lithium carbonate.
TABLE 6CarbonationagentLiPFComposition of mother liquorNa2CO30.40.080.16after carbonation (g / l)K2CO30.20.070.14
TABLE 7CarbonationagentLiPFQuality of crystal of lithium carbonateNa2CO3—(%)K2CO3—
[0114]As shown in Tables 6 and 7, in the carbonation treatment, even when a sodium carbonate (Na2CO3) solution or a potassium carbonate (K2CO3) solution was used as a carbona...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acidity | aaaaa | aaaaa |
| Acidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com