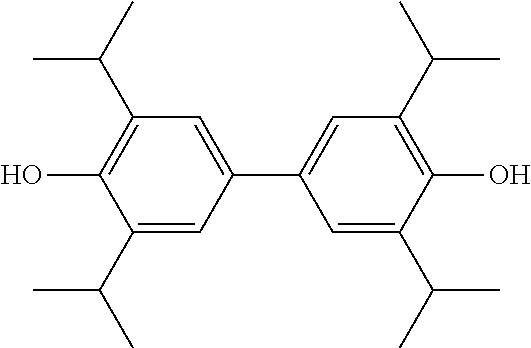
2,2',6,6'-tetraisopropyl-4,4'-biphenol lipid microsphere preparations and preparation methods therefor
a technology of tetraisopropyl and lipid microspheres, which is applied in the field of pharmaceutical preparations, can solve the problems of difficult water dissolution of tetraisopropyl-4,4'-biphenol, limited clinical application, and affect the efficacy, so as to improve the therapeutic effect of the drug and improve the absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1% Biphenol Lipid Microsphere Preparation
[0046]
Drugs and ExcipientsAmount (g)Biphenol10.0Soybean oil (Injection-grade)100Egg lecithin12Vitamin E10Glycerin25EDTA5Injection-grade waterMake up to 1000 ml
Preparation Method
[0047]1) 12 g of egg lecithin was completely dissolved in 100 g of injection-grade oil under a nitrogen atmosphere and in a 70° C. water bath. 10 g of biphenol and 10 g of vitamin E were then added and nitrogen gas was fed in for protection before being dissolved, with heat and stirring, to obtain an oil phase.
[0048]2) 20 g of glycerin and 5 g of EDTA were dissolved, with stirring, in the injection-grade water to obtained an aqueous phase.
[0049]3) The oil phase was added slowly to the aqueous phase while sheared under nitrogen (10000 r, 5 min) to obtain a preliminary emulsion which was then adjusted to around pH 8.0 with sodium hydroxide.
[0050]4) The preliminary emulsion was homogenized with a high-pressure homogenizer and filtered with a microporous membrane filter be...
example 2
0.1% Biphenol Lipid Microsphere Preparation
[0051]
Drugs and ExcipientsAmount (g)Biphenol1.0Sea buckthorn oil (Injection-100grade)Hydrogenated Lecithin12Ascorbic acid10Glycerin25EDTA5Injection-grade waterMake up to 1000 ml
Preparation Method
[0052]1) 12 g of hydrogenated lecithin was completely dissolved in 100 g of injection-grade oil under a nitrogen atmosphere and in a 70° C. water bath. 1 g of biphenol was then added and nitrogen gas was fed in for protection before being dissolved, with heat and stirring, to obtain an oil phase.
[0053]2) 25 g of glycerin, 10 g ascorbic acid and 5 g of EDTA were dissolved, with stirring, in the injection-grade water to obtain the aqueous phase.
[0054]3) The oil phase was added slowly to the aqueous phase while sheared under nitrogen (10000 r, 5 min) to obtain a preliminary emulsion which was then adjusted to around pH 8.0 with sodium hydroxide.
[0055]4) The preliminary emulsion was homogenized with a high-pressure homogenizer and filtered with a microp...
example 3
3% Biphenol Lipid Microsphere Preparation
[0056]
Drugs and ExcipientsAmount (g)Biphenol30Injection-grade Medium-chain100triglyceride oilSoy lecithin12Sodium bisulfite10Glycerin25EDTA5Injection-grade waterMake up to 1000 ml
Preparation Method
[0057]1) 12 g of soy lecithin was completely dissolved in 100 g of injection-grade oil under a nitrogen atmosphere and in a 70° C. water bath. 30 g of biphenol was then added and nitrogen gas was fed in for protection before being dissolved, with heat and stirring, to obtain an oil phase.
[0058]2) 25 g of glycerin, 10 g of sodium bisulfite and 5 g of EDTA were dissolved, with stirring, in the injection-grade water to obtained an aqueous phase.
[0059]3) The oil phase was added slowly to the aqueous phase while sheared under nitrogen (10000 r, 5 min) to obtain a preliminary emulsion which was then adjusted to around pH 8.0 with sodium hydroxide.
[0060]4) The preliminary emulsion was homogenized with a high-pressure homogenizer and filtered with a micropo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com