Irreversible electroporation device and method for attenuating neointimal formation
a neointimal formation, irreversible electroporation technology, applied in the field of medical devices and methods for preventing vascular restenosis, can solve the problems of re-stenosis, re-stenosis rate, and unfavorable re-growth of lesion, so as to avoid thermal damage, and prevent excessive cell lysing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
specific embodiments
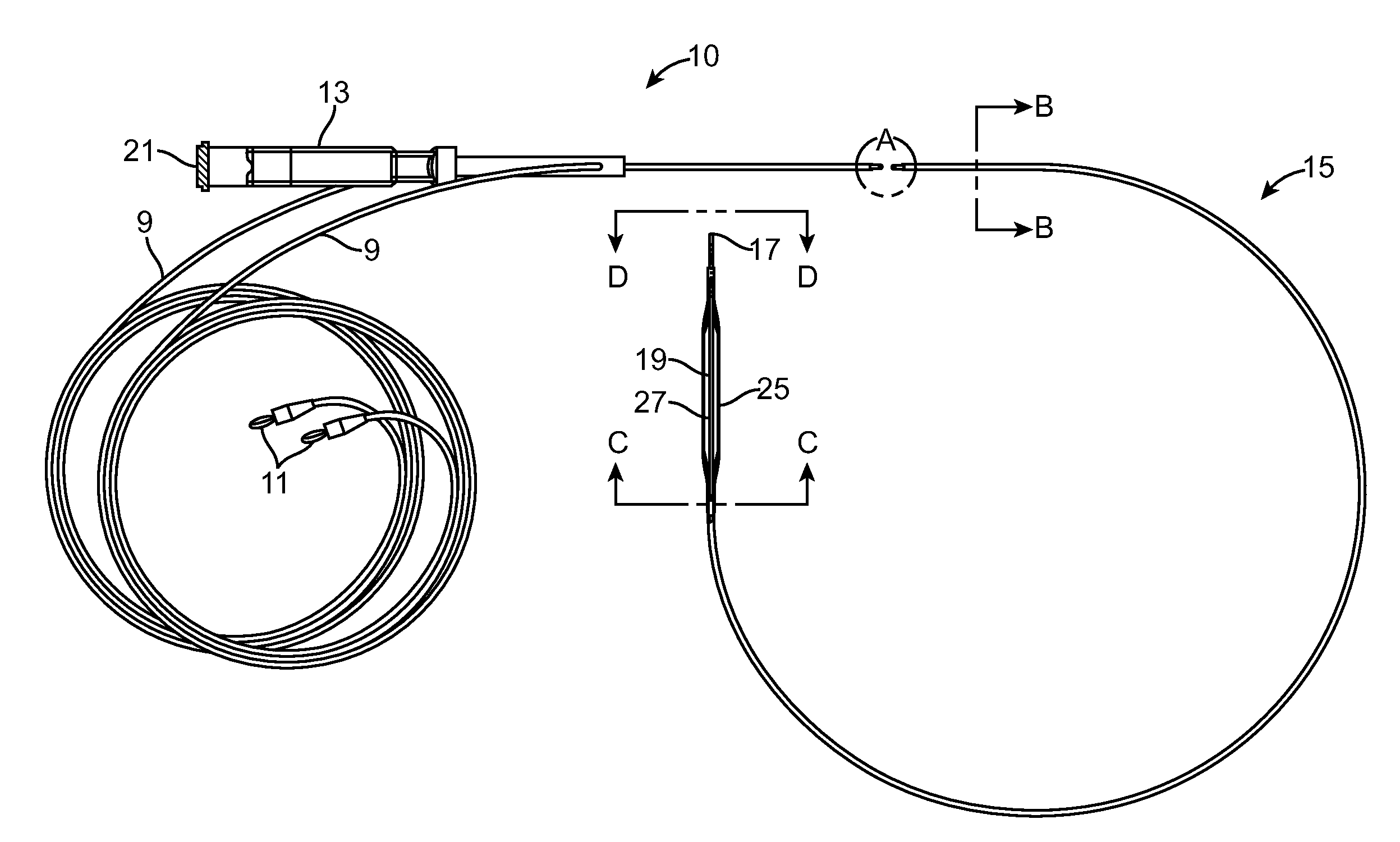
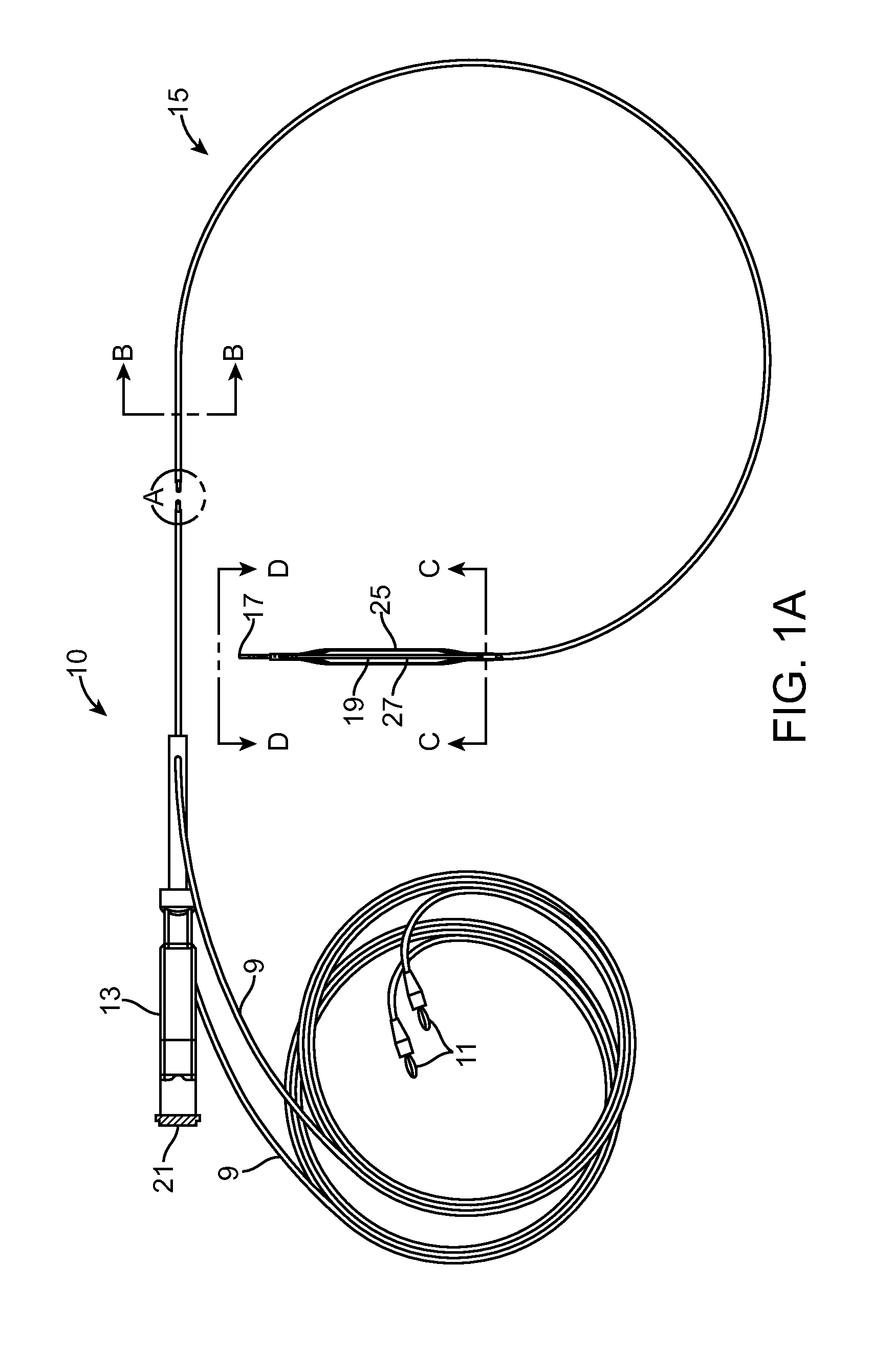
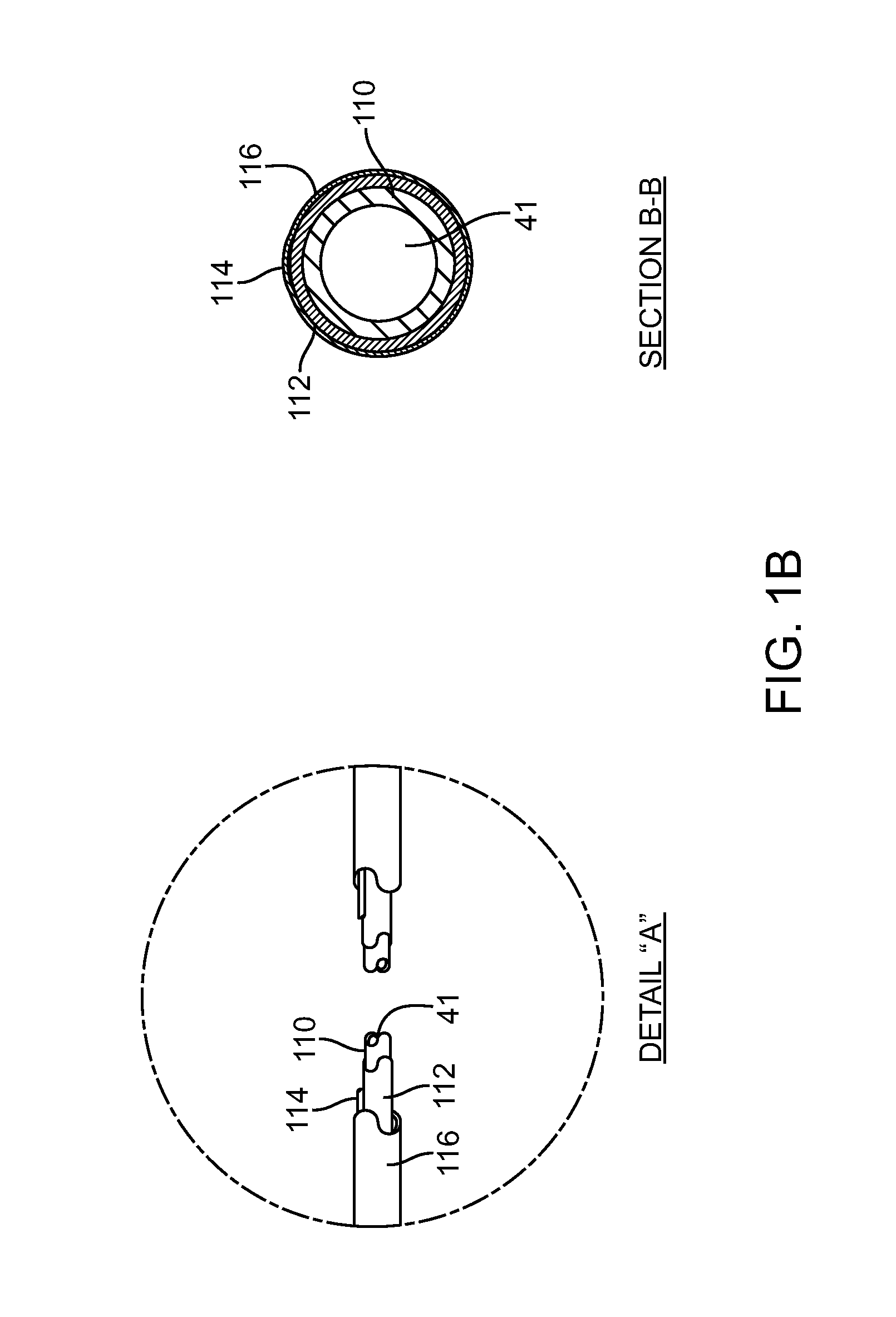
[0078]There are a range of different catheter device type configurations which can be used in connection with the present invention. Some examples of devices which could be modified to obtain the basic objects of the invention include the balloon catheter device of U.S. Pat. No. 7,150,723 teaching a medical device including guidewire and balloon catheter for curing a coronary artery. Another catheter device which might be modified to utilize the aspects of the invention is the device of U.S. Pat. No. 7,273,487 disclosing a balloon catheter having a multi-layered shaft with variable flexibility. Still another balloon catheter device is taught within U.S. Pat. No. 7,351,214 disclosing a steerable balloon catheter. Yet another device is taught within U.S. Pat. No. 7,481,800 disclosing a triple lumen stone balloon catheter and method. The present invention is not specific to any of these embodiments and other embodiments can be used to provide various catheter configurations which inclu...
example 1
Method
[0130]Eight Sprague-Dawley rats weighting 300-350 grams were used in this pilot study. All animals received humane care from a properly trained professional in compliance with both the Principals of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals, published by the National Institute of Health (NIH publication No. 85-23, revised 1985).
[0131]Each animal was anaesthetized throughout the procedure. The left common carotid artery was exposed, and intimal denudation was performed as previously described. Naor, et al. “The Effect of Irreversible Electroporation on Blood Vessels”Technol Cancer Res Treat. 6, 2007: 255-360; Touchard, et al. “Preclinical Restenosis Models Challenges and Successes,”Toxicologic Pathology, 34, pp. 2006: 11-181 Briefly, the left external carotid artery was incised, and a 2F Fogarty arterial embolectomy catheter (Edwards Lifesciences) was advanced through the incision to the left common carotid artery. The balloon was inflated ...
example 2
Summary of Method and Results
[0153]33 Sprague-Dawley rats were used to compare NTIRE protocols. Each animal had NTIRE applied to its left common carotid using custom-made electrodes. The right carotid artery was used as control. Electric pulses of 100 microseconds were used. Eight IRE protocols were compared: 1-4) 10 pulses at a frequency of 10 Hz with electric fields of 3500, 1750, 875 and 437.5 V / cm and 5-8) 45 and 90 pulses at a frequency of 1 Hz with electric fields of 1750 and 875 V / cm. Animals were euthanized after one week. Histological analysis included VSMC counting and morphometry of 152 sections. Selective slides were stained with elastic Van Gieson and Masson trichrome to evaluate extra-cellular structures. Most efficient protocols were 10 pulses of 3500 V / cm at a frequency of 10 Hz and 90 pulses of 1750 V / cm at a frequency of 1 Hz, with ablation efficiency of 89±16% and 94±9% respectively. Extra-cellular structures were not damaged and the endothelial layer recovered co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com