Novel synthesis of galactoside inhibitors
a technology of galactoside inhibitors and synthesis routes, which is applied in the field of new synthesis routes for the manufacture of galactoside inhibitors, can solve the problems of increasing the amount of unwanted azide reduction, and achieve the effect of efficient manufactur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
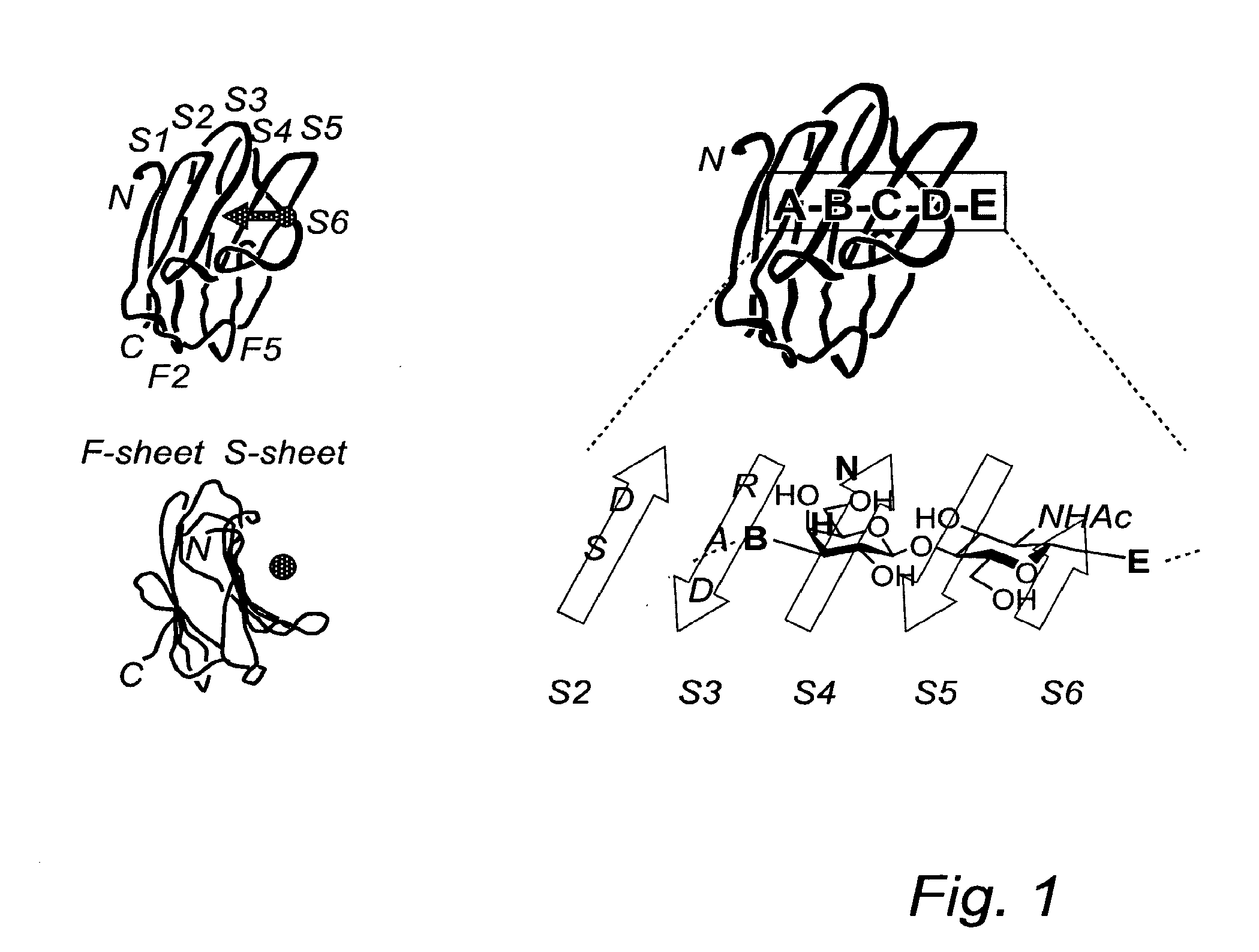
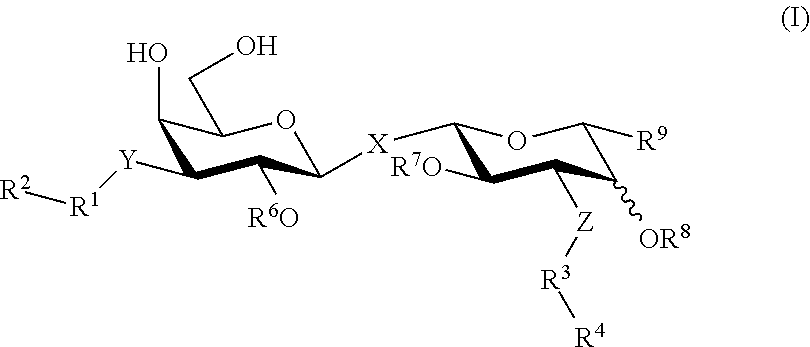
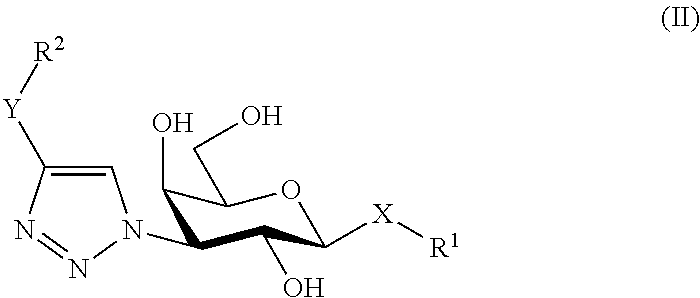
[0078]The novel route of synthesis is clearly shown in scheme 1 below, and relates in particular to the reaction of compounds of formulae (8) and (9) in the final step to form a compound of formula (10), which thiodigalactoside compound is further deprotected to form compound of formula (II) and then further reacted to form the compounds of formula (12) or (13).
[0079]In a further aspect of the invention it relates to intermediates as well, in particular the intermediates of the compounds (2) to (7) and (9) above.
[0080]The phenyl group present on the acetal carbon may be substituted with a methyl, methoxy, alkyl, alkoxy, or aryl or fused with an aryl group as well.
[0081]The phenyl group present on the S-atom, the thio group, may be substituted with a methyl, methoxy, alkyl, alkoxy, halo, nitro, or amido group as well.
[0082]In addition to acetates, the esters of compounds (2) to (10) may be aliphatic esters of 1 to 6 carbon atoms, aromatic esters, or substituted aromatic ester.
[0083]A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
| stress | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com