Fermentation process for the production of organic acids
a fermentation process and organic acid technology, applied in the direction of fermentation, etc., can solve the problems of carbon dioxide gas release into the atmosphere, inefficient use of carbon dioxide gas as a source of inorganic carbon in the fermentation solution,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Solid Inorganic Carbon Source
[0083]A stock solution containing both ammonium hydroxide and ammonium bicarbonate was prepared by means of sequestering carbon dioxide in the solution of ammonium hydroxide (FIG. 1). One liter of 28-30% ammonium hydroxide solution was added to a 3 liter NBS (New Brunswick Scientific) fermentor and carbon dioxide gas was micro-sparged at the rate of 1 L / minute. The ammonium hydroxide solution inside the fermentor was stirred at 500 rpm for an hour. At the end of one hour, the temperature of the fermentor had increased to 39.3° C. from an initial temperature of 19.5° C. Cooling of the fermentor was initiated by circulating cold water through a coil within the fermentor. With the cold water circulation the temperature of the fermentor reached 16.2° C. in about 2 hours. When the solution turned into a white slimy liquid, 325 ml of water was added to obtain approximately 11 M combined solution of ammonium with approximately 3 M bicarbonate. 11...
example 2
Succinic Acid Production with NH4OH and NH4HCO3
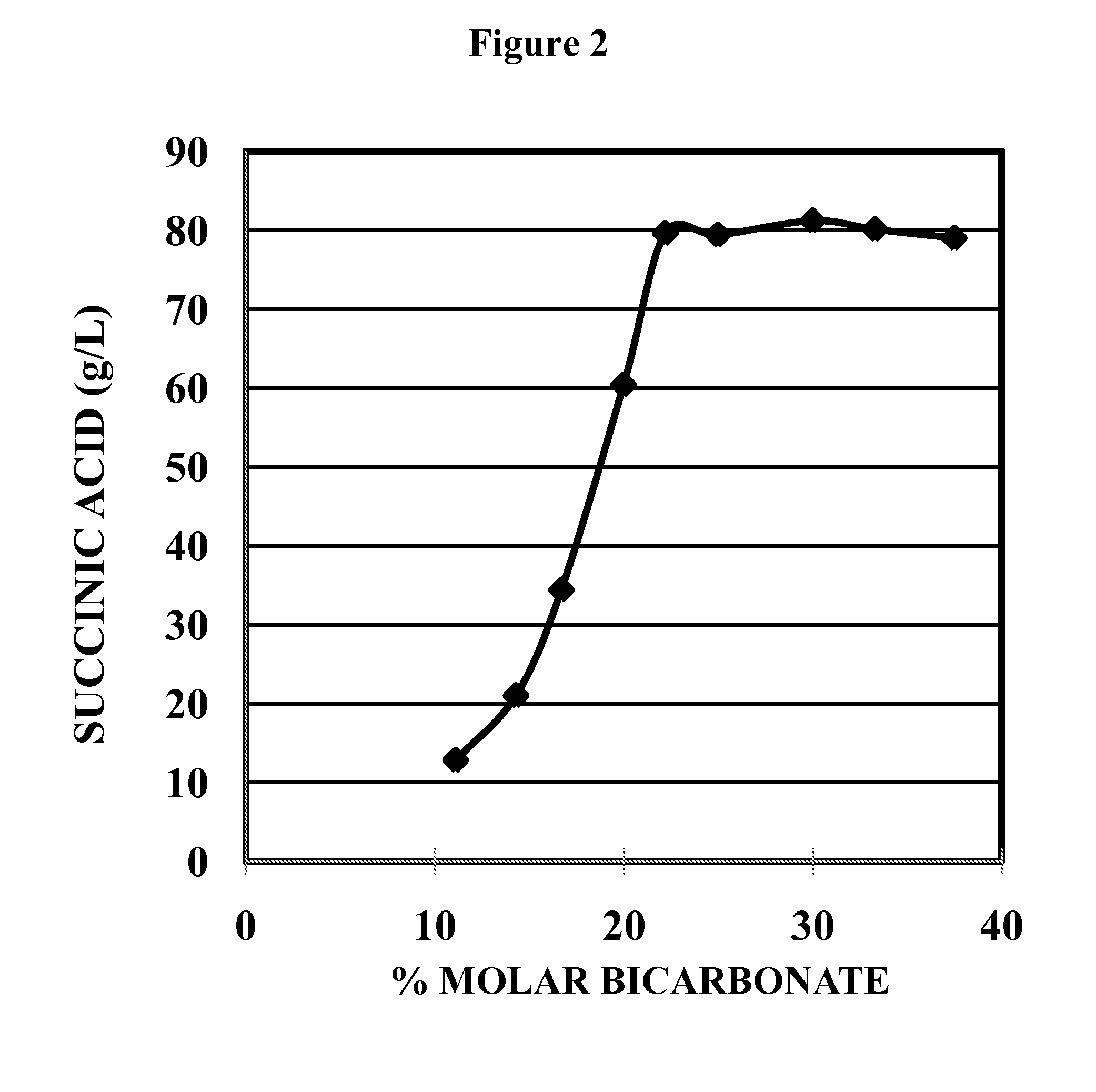
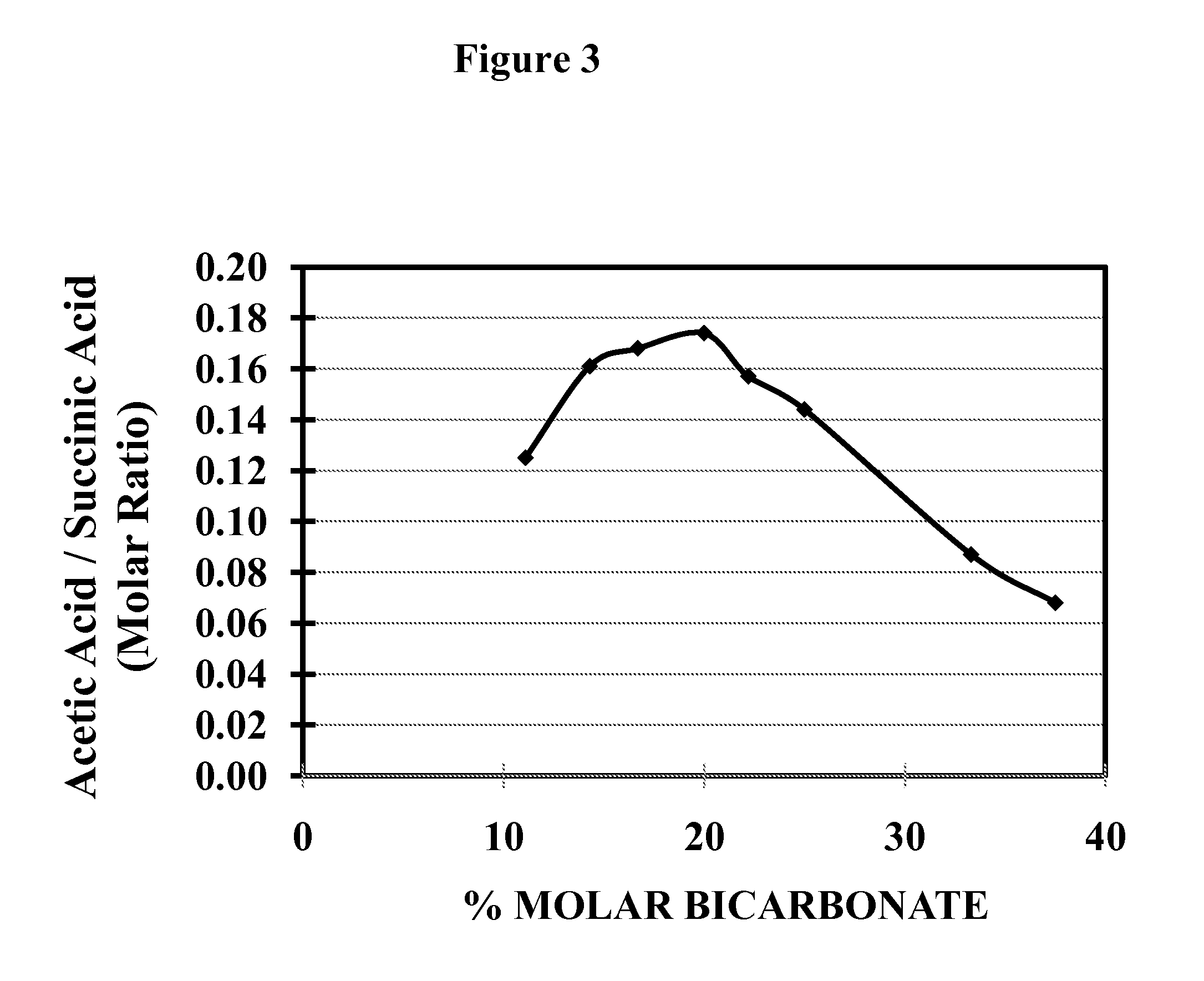
[0085]In order to identify the optimal ratio for NH4OH and NH4HCO3 in the fermentative production of succinic acid, a series of succinic acid fermentations were conducted with varying ratios of NH4OH and NH4HCO3. As shown in the Table 1 below, twelve different NH4OH—NH4HCO3 compositions were tested in the succinic acid fermentation using the KJ122 strain of E. coli as a biocatalyst in a total volume of 2,000 ml in a NBS fermentor maintained at 39° C. The fermentation medium also contained KH2PO4 (55 ml of 1M KH2PO4), MgSO4 (4 ml of 1.5 M MgSO4), betaine (4 ml of 1M betaine). and trace elements. KJ122 inoculum had an initial OD550 nm of 6.8 to 7.8 and 150 ml of this inoculum representing 7.5% (v / v) of the total fermentation volume was used. The pH was maintained at 6.5 and fermentation fluid was stirred with the impeller within the fermentor operated at 750 RPM. Glucose solution was fed as required. At the end of the fermentation the ti...
example 3
Potassium Requirement in Succinic Acid Production
[0086]In this study efforts were made to determine whether potassium salts could be entirely eliminated from the fermentation medium without any significant effect on the succinic acid productivity. In the control experiment, the fermentation was carried out with an initial volume of 4,000 ml in AM1 medium using KJ122 as a biocatalyst. 3N NH4OH and 0.75 M K2CO3, and 1.5 N KOH were used as the neutralizing base. Glucose was provided at the initial concentration of 102.9 grams per liter. At the end of 38 hours of fermentation, the glucose was completely utilized. At the end of 38 hours of fermentation, the succinic acid productivity was calculated to be 1.45 g / L / hr. In the second experiment, fermentation was carried out with 6N NH4OH as the only neutralizing base in an initial volume of 2,000 ml. K2CO3 and KOH were completely eliminated from the fermentation medium. Carbon dioxide gas was provided as the source of inorganic carbon at th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com