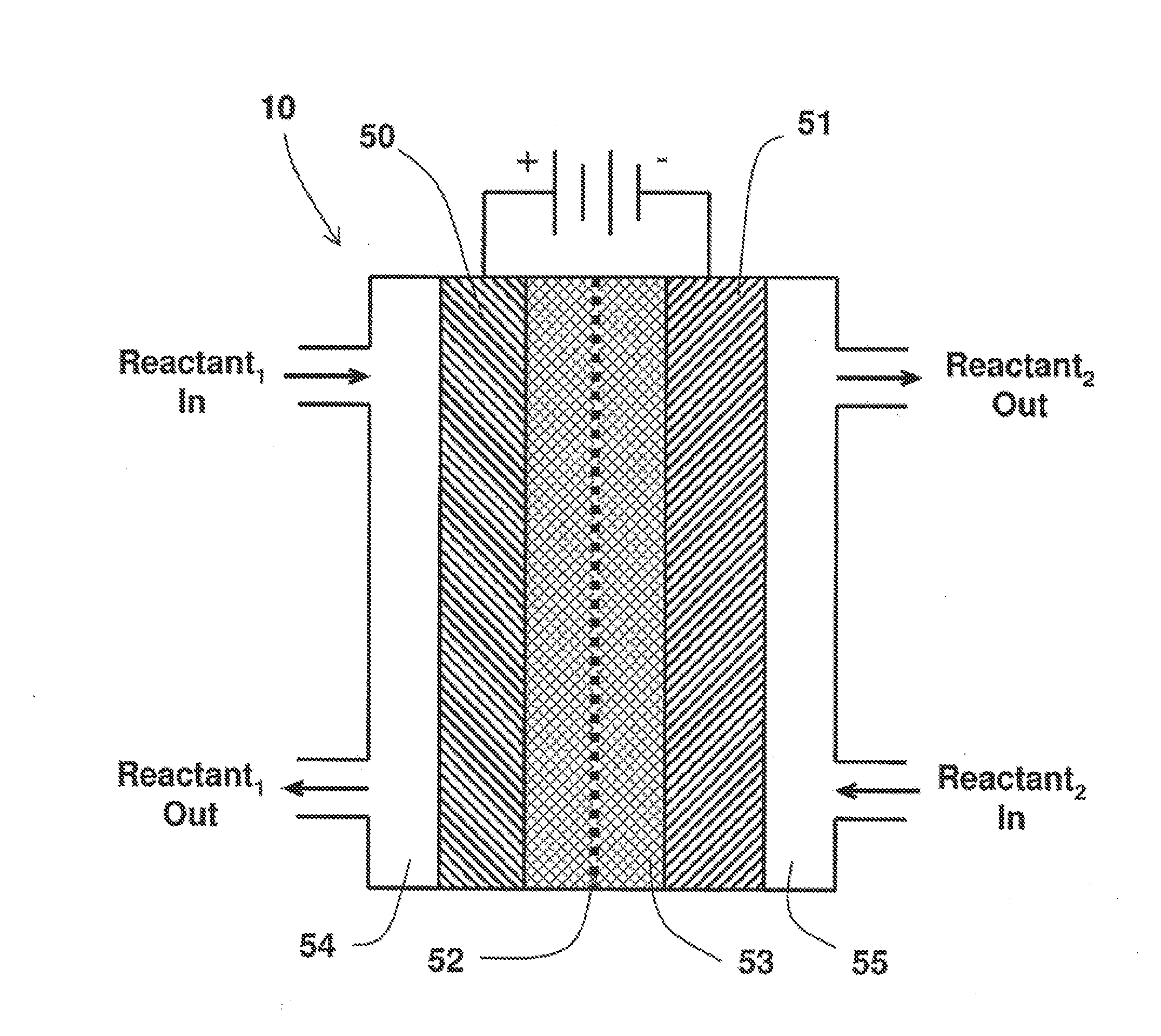
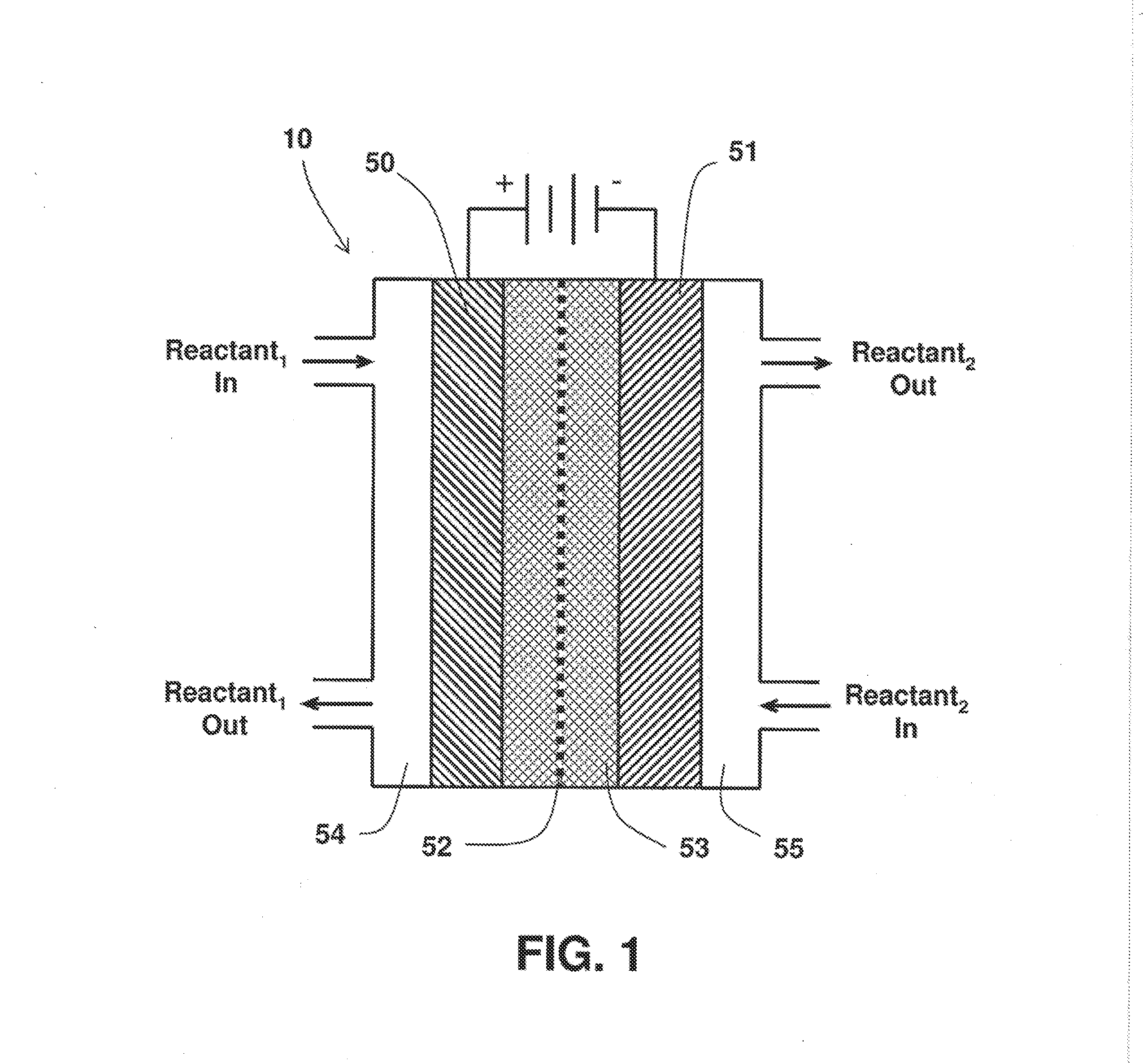
On Demand Carbon Monoxide Generator For Therapeutic and Other Applications
a carbon monoxide generator and generator technology, applied in the field of carbon monoxide generator on demand, can solve the problems of poisonous carbon monoxide, hazardous conditions, and inconvenient delivery of cylinders in clinical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
specific example 1
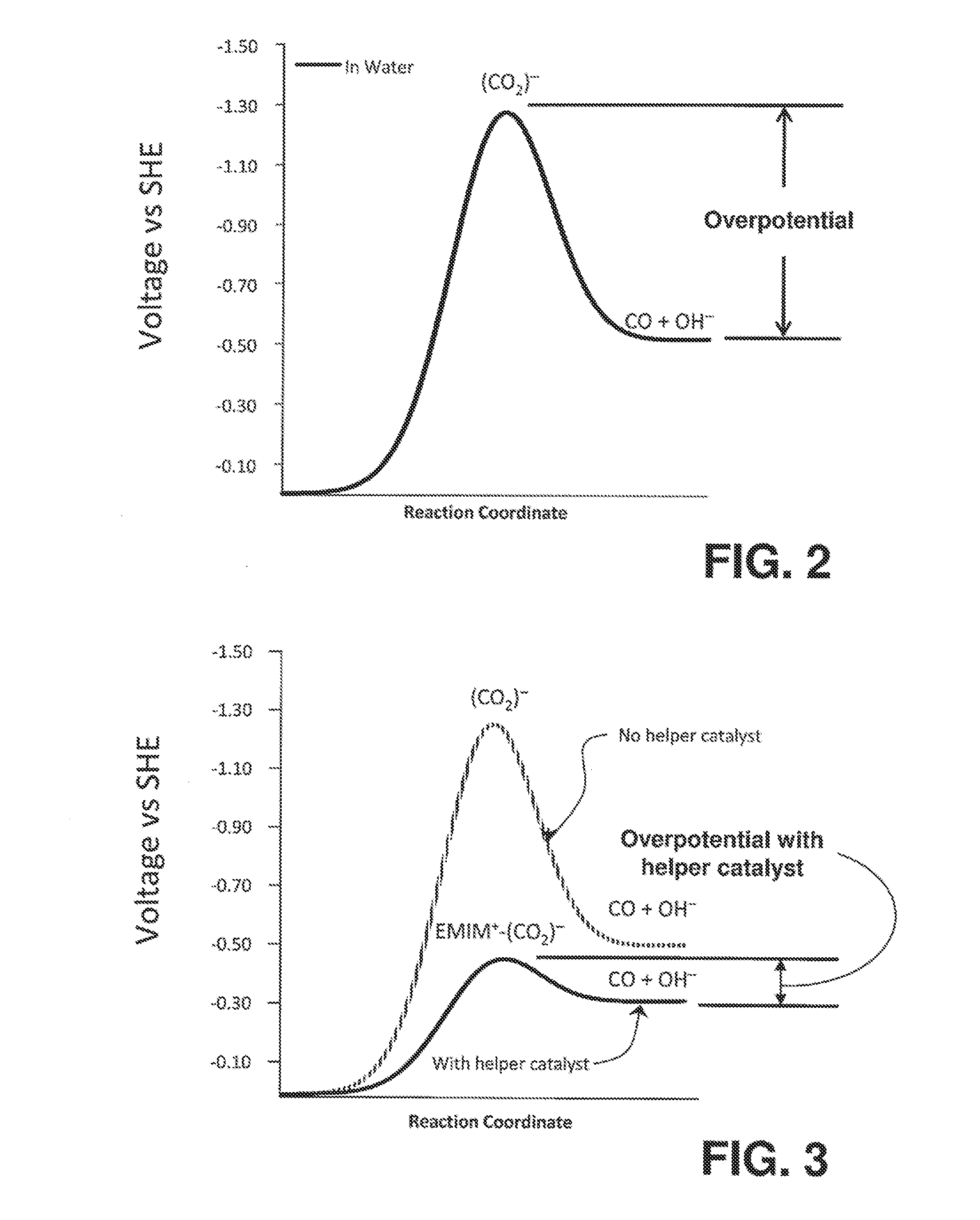
[0096]The following section describes the testing procedure used for an Active Element, Helper Catalyst Mixture as previously disclosed in the related applications cited above. These particular experiments measured the ability of an Active Element, Helper Catalyst Mixture consisting of platinum and 1-ethyl-3-methylimidazolium tetrafluoroborate (EMIM-BF4) to lower the overpotential for electrochemical conversion of CO2 to CO and raise the selectivity (current efficiency) of the reaction. Therefore, the test can determine whether EMIM-BF4 and the EMIM+ ion can serve as director molecules and director ions, respectively, for the desired reaction. The desired reaction in this test will be the electrochemical reduction of carbon dioxide (typically to primary products such as CO).
[0097]The experiments used the glass three electrode cell shown in FIG. 7. The cell consisted of a three neck flask 101, to hold the anode 108, and the cathode 109. Seal 107 forms a seal around anode wire 108. Fi...
specific example 2
Steady State Production of Carbon Monoxide
[0109]This experiment used the flow cell described in Devin T. Whipple, E. C. Finke, and P. J. A. Kenis, Electrochem. & Solid-State Lett., 2010, 13 (9), B109-B111 (“the Whipple paper”). First, catalyst inks were prepared as follows:
[0110]For the cathode: 10 mg of silver nanoparticles (Sigma Aldrich) was sonicated into a solution containing 100 μL of water, 100 μL of isopropyl alcohol and 5.6 μL of 5% perfluorosulfonic acid solution (available under the trade designation Nafion, from Ion Power, Inc., New Castle, Del., USA). The resultant catalyst ink was painted on a 1×1.5 cm section of a 2×3 cm piece of carbon paper (Ion Power, Inc.) and dried with a heat lamp.
[0111]The preparation was identical for anode except 4 mg of HiSpec 1000 platinum black (Sigma Adrich) was substituted for the silver.
[0112]Both catalysts were mounted in the flow cell described in the Whipple Paper. Five sccm of CO2 was fed to the anode, and a solution containing 18 m...
specific example 3
High Quality Carbon Monoxide Production Over a Wide Range of Rates
[0114]Example 2 showed that CO could be produced at high rates and selectivities, but when the voltage was decreased, so the rate decreased, the CO2 to hydrogen ratio was less than 20. This could create a problem in clinical systems where there is a need to produce carbon monoxide over a wide range of rates. This example describes a modified design that allows one to produce pure carbon monoxide over a wider range of conditions.
[0115]The apparatus and procedures were the same as in Specific Example 2, except that a Nafion 117 membrane (available from Ion Power, Inc.) was inserted between the cathode and the anode to create separate anode and cathode compartments. The anode compartment contained 100 mM aqueous sulfuric acid flowing at 0.5 ml / min. The cathode compartment contained 18 mol % EMIM-BF4 in water at 0.5 ml / min. A potential was applied to the cell, and the data in FIG. 11 were measured with a gas chromatograph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| corrosive | aaaaa | aaaaa |
| purities | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com