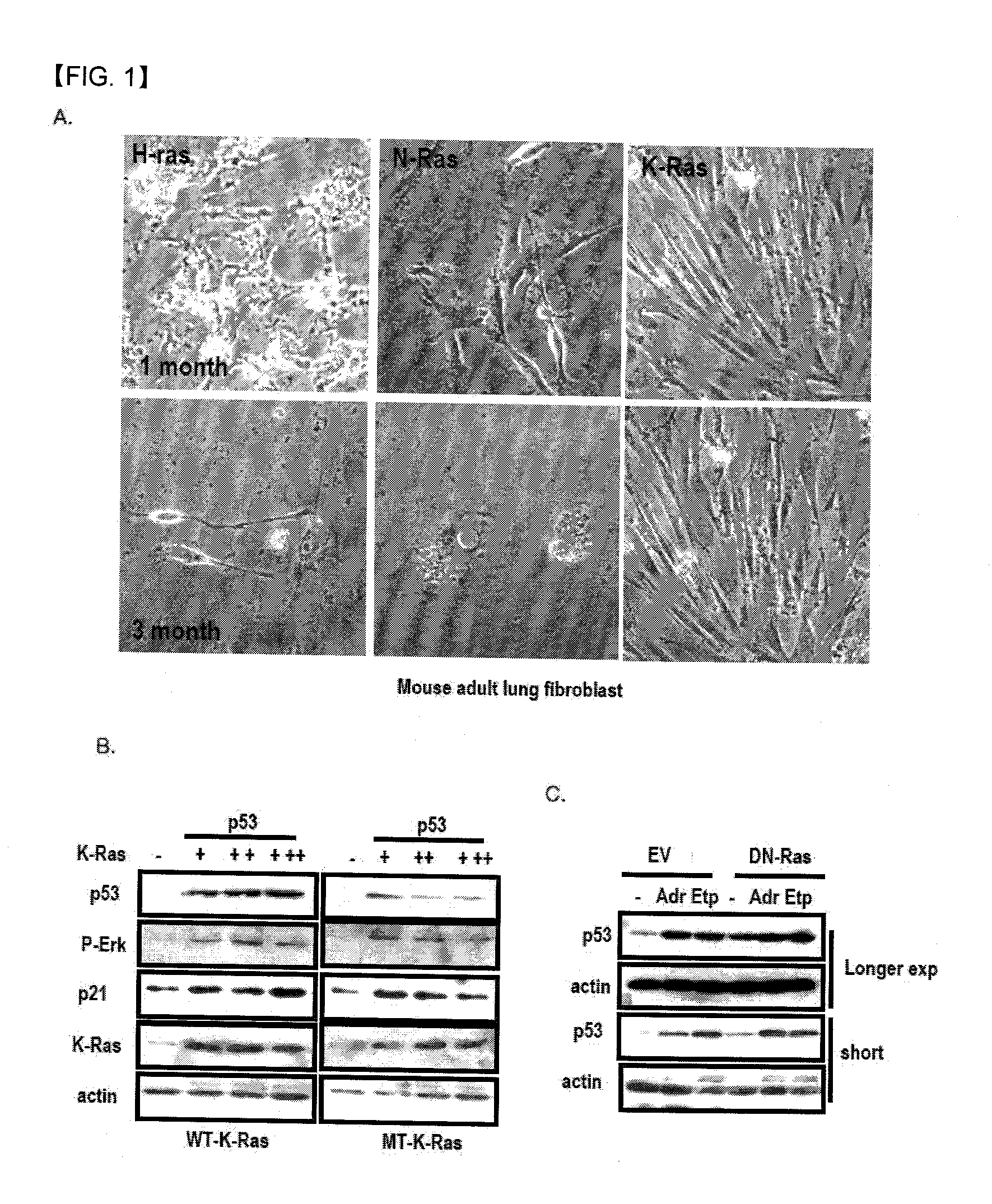
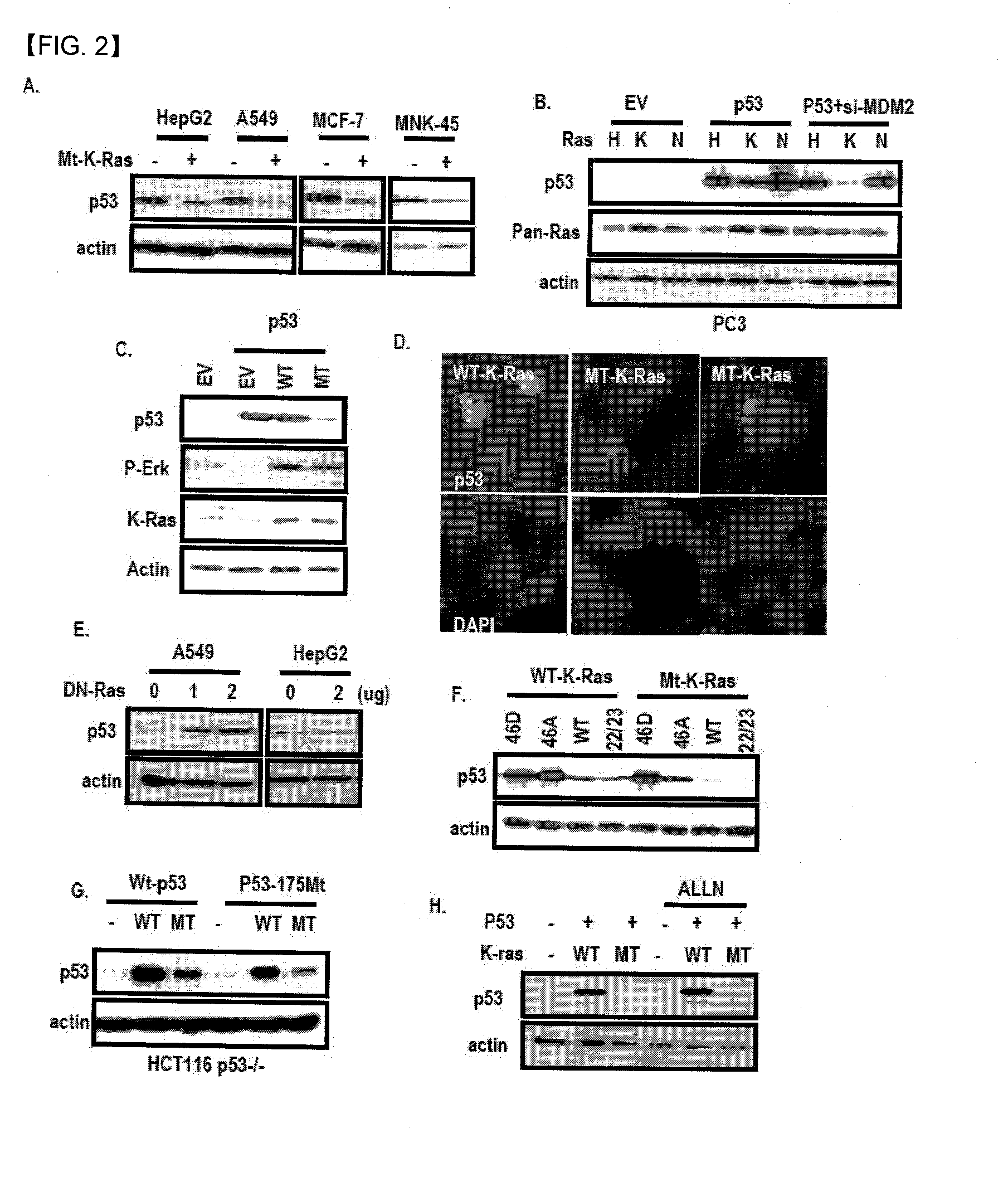
Chemical inhibitor of p53-snail binding and pharmaceutical composition for treating cancer disease containing same as its active ingredient
a technology of p53snail binding and pharmaceutical composition, which is applied in the direction of drug compositions, ester active ingredients, amide active ingredients, etc., can solve the problems of prolonging life span, less than 10%, and low survival rate of lung and pancreatic cancer, so as to improve the survival rate of caner patients or treatment efficiency, the effect of effective treatment or preventing a k-ras mutant cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 6
[0126]1. Behaviors of Extracellular p53 and Snail
[0127]1) Tissue Analysis
[0128]Normal and tumor paired cholangioma and liver tissues were obtained from Shunchunhyang Medical Center. Tissues were rapidly frozen in the deep freezer until use. Frozen tissues were sliced and 0.5 mg of tissues was incubated in 0.25 ml serum free medium for 30 min. at 37° C. to allow the release of tissue fluid. After incubation, the culture medium was collected and precipitated with 0.5 ml 100% Et-OH. Precipitated materials were dissolved using RIPA and used for SDS-PAGE and WB analysis. We also obtained same culture medium through the same method and used the culture medium to detect p53 antibody.
[0129]2) p53 ELISA Assay
[0130]To examine the p53, we performed the ELISA following manufacture's protocol (Assay Design). In brief, 0.2 ml tissue cultured media was added to wells and incubated with detection antibody. After washing with a wash buffer, 0.2 ml of a substrate sol and 0.05 ml of a stop solution we...
example 1
1-1. Synthesis of 2-methylthio-1,4-naphthoquinone (1a)
[0143]0.617 mM 1,4-naphtoquinone was dissolved in 30 ml of methanol in 100 ml one-neck round flask, and 1.54 mM sodium thiomethoxide was added thereto and stirred overnight. 50 ml of saturated sodium chloride solution was added to the reaction mixture, followed by extraction three times with 50 ml of chloroform, and an organic layer was dehydrated with an anhydrous sodium sulfate and filtered. The filtrate was concentrated under reduced pressure and the obtained residue was re-crystallized with methanol to produce 2-methylthio-1,4-naphthoquinone that was yellow crystal.
[0144]Yield: 14.0%, melting point: 185-186° C., 1H-NMR (CDCl3, 400 MHz): δ 8.12-8.07(m, 2H), 7.78-7.70(m, 2H), 6.58(s, 1H), 2.40(s, 3H), m / z 205.1 (M+H)+.
1-2. Synthesis of 2-ethylthio-1,4-naphthoquinone (1b)
[0145]2-ethylthio-1,4-naphthoquinone that was yellow crystal was prepared in the same manner as in Example 1-1, except that ethylmercaptan was used instead of s...
example 2
2-1. Synthesis of 2-methylamino-5,8-dimethoxy-1,4-naphthoquinone (5a)
[0163]0.45 mM 5,8-dimethoxy-1,4-naphthoquinone (4) which had been prepared above was dissolved in 30 ml of methanol in 100 ml one-neck round flask and then, 0.687 mmol methylamine was added thereto and stirred at room temperature for 3 hours 0.64 mM sodium dichromate and 0.18 mM sulfuric acid dissolved in water were slowly dropped to the reaction mixture and stirred at room temperature for 3 minutes. Then, 50 ml of saturated sodium chloride was added to the reaction mixture, followed by extraction three times with 50 ml of chloroform and obtained organic layers were gathered and dehydrated with anhydrous sodium sulfate and filtered. The filtrate was concentrated under reduced pressure and the residue was subjected to silicagel column chromatography, thereby producing 2-methylamino-5,8-dimethoxy-1,4-naphthoquinone that was reddish brown. The yield and properties of the synthesized compound are as follows.
[0164]Yield...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| permeation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com