Moesin modulators and uses thereof
a moesin and modulator technology, applied in the field of moesin modulator and moesin activity, can solve the problems of unreliable correlation between clinical manifestation and anti-erm antibodies, and achieve the effect of effectively treating or preventing mammal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of the Anti-moesin Antibodies
[0129]Monoclonal antibody against the C-terminal tail domain of moesin was prepared by using the conventional hybridoma methods. To generate the C-terminal domain having the sequence of SEQ ID NO:1, PCR was used to amplify cDNA fragments corresponding to the C-terminal tail domain as described above (see SEQ ID NO:4 shown in FIG. 1B, wherein the underlined portion is the cDNA sequence of the C-terminal tail domain).
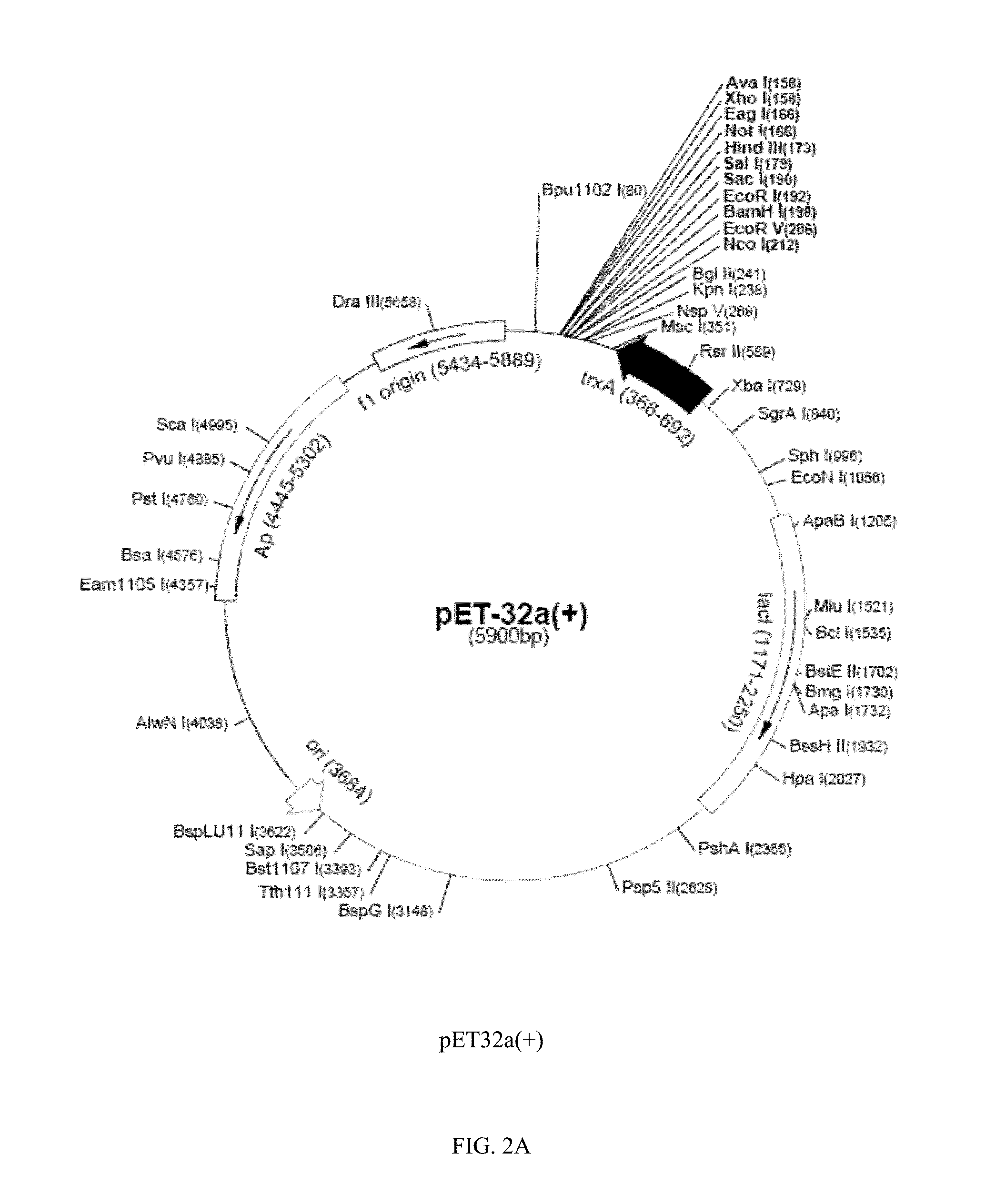
[0130]PCR-amplified moesin DNA fragments were cloned into expression vectors selected from pET32a(+) and pET28a(+). The constructed vectors were then used to transform E. coli host cell line BL21(DE3) for culturing and expression. The restriction and cloning maps of pET32a(+) and pET28a(+) are shown in FIGS. 2A and 2B, respectively. The constructed expression systems for the C-terminal domain were verified with restriction enzyme digestion followed by sequencing to confirm the correct reading frame for expression of the C-terminal ...
example 2
Assessing Moesin Inhibitor's Ability to Inhibit Cell Proliferations
[0135]This experiment is used to assess moesin inhibitor's ability to inhibit or reduce cell proliferation.
[0136]Cell proliferation assay were performed using a human pulmonary microvascular endothelial cell line (HPMEC). Cells were plated in each well on a 6-well plate at 106 cells / cm2, and cultured at room temperature in the presence of various testing and control reagents as described below. After culturing for a determined period of time, cells were collected and labeled for flow cytometry analysis. Proliferation rates at 2 hrs, 24 hrs and 36 hrs were determined by dividing the mean OD570 value from the tested groups with the mean OD570 value from the group having the same number of cells as the test groups at the beginning of the cell culturing.
[0137]Tested and control groups are as follows:[0138]1) TNF-alpha alone;[0139]2) Antibody against full length moesin protein (anti-Moesin);[0140]3) Antibody against the C...
example 3
Analysis of Intercellular Expression of Moesin and Apoptosis
[0145]Cell cultures in the presence of anti-moesin antibodies as described in Example 1 were subjected to apoptosis assay as well as surface antigen assay, to assess the antibody's effect on promoting endothelial cell apoptosis and on intercellular expression of moesin (indicating the active form of the protein).
[0146]Annexin V assay was used to study apoptosis. Collected cells were washed with PBS, centrifuged, and added sequentially with 70% ethanol, RNAs (200 mg / 1) and PI (20 mg / 1). Cells were then stained with Annexin-V FITC / PI kit for double staining of FITC and PI, because viable cells are both FITC and PI negative, while cells that are in early apoptosis are FITC positive but PI negative, and cells that are in late apoptosis or already dead are both FITC and PI positive. Stained cells are analyzed using a flow cytometer for amount of apoptotic cells in the presence of variant testing agents. The percentage of cells i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| composition | aaaaa | aaaaa |
| cell-surface structures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com