Benzazepine derivatives useful as vasopressin antagonists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
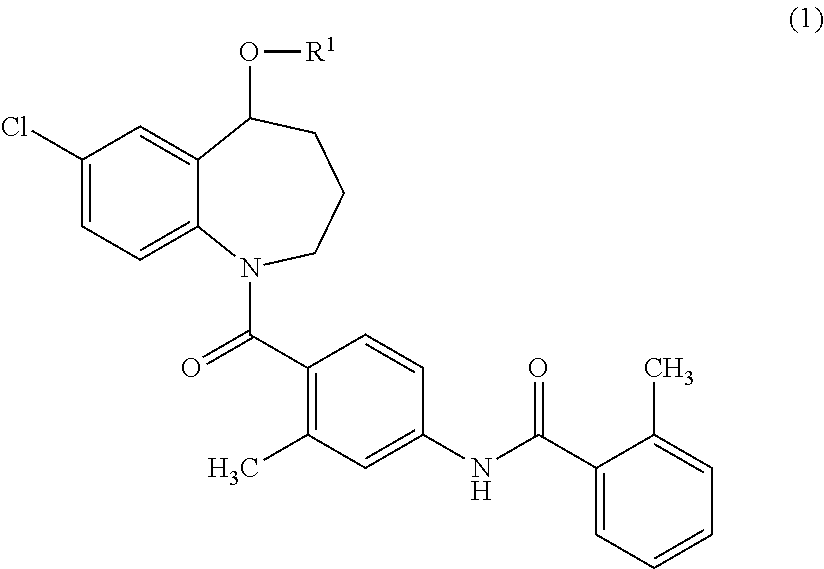
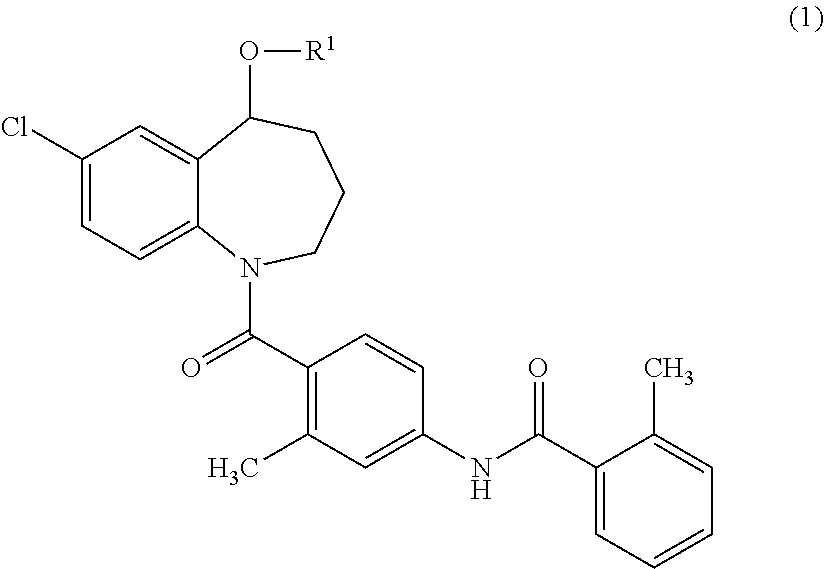
{7-Chloro-1-[2-methyl-4-(2-methyl-benzoylamino)-benzoyl]-2,3,4,5-tetrahydro-1H-benzo[b]azepin-5-yl}succinate
[0148]Tolvaptan (1.0 g, 2.2 mmol), succinic anhydride (0.33 g, 3.3 mmol), and 4-dimethylaminopyridine (DMAP) (27 mg, 0.22 mmol) were added to 1-methyl-2-pyrolidone (3 ml), and the mixture was stirred at 100° C. for 1 hour. Water was added to the reaction mixture, and the resulted precipitates were collected by filtration. The precipitates were purified using silica gel flash chromatography (n-hexane:ethyl acetate=50:50→20:80). The purified product was concentrated under reduced pressure. The residue was dissolved in aqueous acetonitrile, and then freeze-dried to obtain 300 mg of {7-chloro-1-[2-methyl-4-(2-methyl-benzoylamino)-benzoyl]-2,3,4,5-tetrahydro-1H-benzo[b]azepin-5-yl}succinate as white amorphous solid.
[0149]1H-NMR (DMSO-d6, 100° C.) δ ppm:
[0150]1.6-2.1 (4H, m), 2.37 (6H, s), 2.5-2.6 (2H, m), 2.6-2.7 (2H, m), 3.0-4.3 (2H, m), 5.9-6.0 (1H, m), 6.8-7.1 (2H, m), 7.1-7.3 (...
example 2
Sodium {7-chloro-1-[2-methyl-4-(2-methylbenzoylamino)benzoyl]-2,3,4,5-tetrahydro-1H-benzo[b]azepin-5-yl}succinate
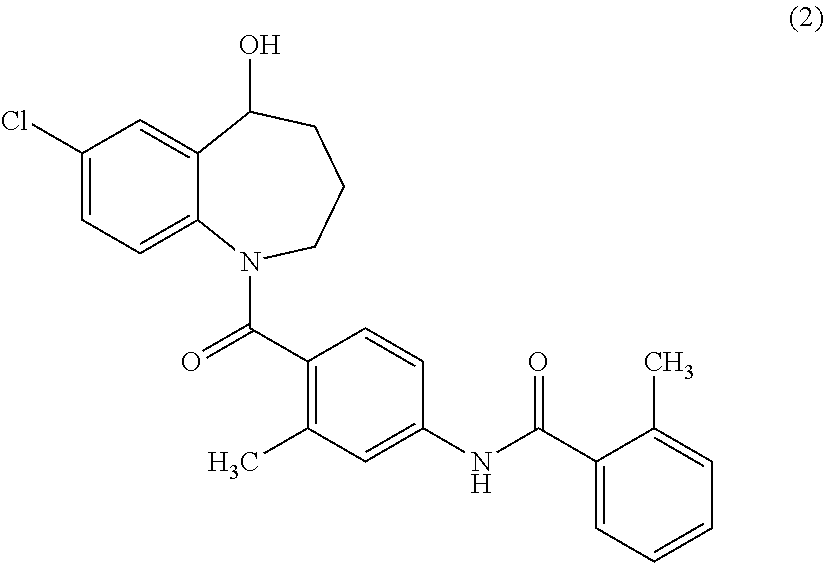
[0151]A sodium hydrogen carbonate (46 mg, 0.55 mmol) aqueous solution (2 ml) was added to a methanol solution (2 ml) of {7-chloro-1-[2-methyl-4-(2-methylbenzoylamino)benzoyl]-2,3,4,5-tetrahydro-1H-benzo[b]azepin-5-yl}succinate (0.30 g, 0.55 mmol), and the mixture was stirred at room temperature for 1 hour. Methanol was distilled off under reduced pressure at about 30° C. The resulting solution was freeze-dried to obtain 0.29 g (94%) of sodium {7-chloro-1-[2-methyl-4-(2-methylbenzoylamino)benzoyl]-2,3,4,5-tetrahydro-1H-benzo[b]azepin-5-yl}succinate as amorphous.
[0152]1H-NMR (DMSO-d6, 100° C.) δ ppm:
[0153]1.70-2.10 (4H, m), 2.19 (2H, t, J=7.1 Hz), 2.37 (6H, s), 2.56 (2H, t, J=7.1 Hz), 3.05-3.50 (1H, m), 3.65-4.25 (1H, m), 5.85-5.95 (1H, m), 6.75-6.90 (1H, m), 6.90-7.10 (2H, m), 7.15-7.55 (6H, m), 7.58 (1H, s), 9.80 (1H, br).
example 3
Potassium {7-chloro-1-[2-methyl-4-(2-methylbenzoylamino)benzoyl]-2,3,4,5-tetrahydro-1H-benzo[b]azepin-5-yl}succinate
[0154]Amorphous of potassium {7-chloro-1-[2-methyl-4-(2-methylbenzoylamino)benzoyl]-2,3,4,5-tetrahydro-1H-benzo[b]azepin-5-yl}succinate was obtained in a similar manner as in the above Example 2.
[0155]1H-NMR (DMSO-d6, 100° C.) δ ppm:
[0156]1.70-2.10 (4H, m), 2.16 (2H, t, J=7.1 Hz), 2.37 (6H, s), 2.48 (2H, t, J=7.1 Hz), 2.95-3.50 (1H, m), 3.70-4.25 (1H, m), 5.85-5.95 (1H, m), 6.75-6.90 (1H, m), 7.00-7.15 (2H, m), 7.20-7.45 (6H, m), 7.58 (1H, s), 9.77 (1H, br).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com