Method of fixing and expressing physiologically active substance
a technology of physiologically active substances and re-injection, which is applied in the field of methods for retaining and expressing physiologically active substances, can solve the problems of inability to work, inability to retain introduced nucleic acids, and rapid degradation, and achieves safe and effective retention, increased clinical feasibility of pharmaceutical agents, and little systemic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0108]DSS enteritis was induced by allowing 12-week-old male Wistar rats to freely drink water containing 3% dextran sulfate sodium (DSS) (molecular weight; 50,000) (Okayasu I, Hatakeyama S, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98: 694-702). An ultrathin endoscope for humans was inserted into the large intestines of rats anesthetized with Nembutal on day 0 and 3 after the start of feeding with DSS water. After observation of the mucosa, siRNA was injected into the submucous tissue at equally spaced four sites in the left colon. The ultrathin endoscope used was a prototype model (outer diameter of the scope, 5.6 mm) having a working forceps channel (channel diameter, 2 mm) which had been developed as an upper gastrointestinal endoscope for humans by OLYMPUS. Untreated rats were used as a control.
[0109]The therapeutic effect was evaluated based on: (1) clinical disea...
example 2
Retention of Physiologically Active Substances in the Submucous Tissue of Rat Large Intestine
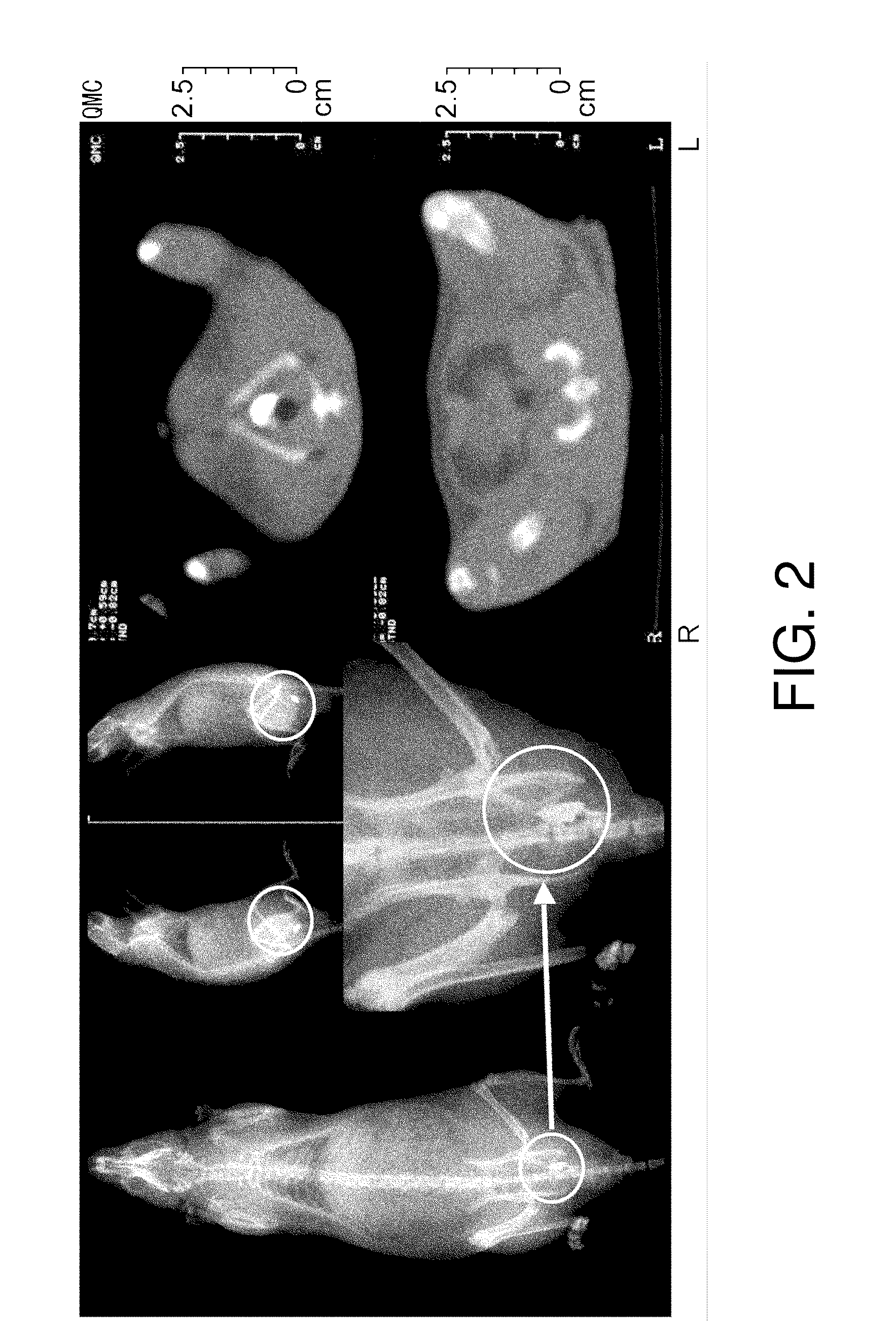
[0110]Next, the retention of physiologically active substances in the submucous tissue of normal rats was analyzed by diagnostic imaging using X-ray and CT, which are routinely used clinically. Twelve-week-old male Wistar rats were anesthetized with Nembutal, and then an ultrathin endoscope for humans was inserted into the large intestines and 20 μl of iopamidol, a contrast medium, was endoscopically injected alone into the submucous tissue of the left colon using a local injection needle.
[0111]Iopamidol refers to the compound named N,N′-Bis[2-hydroxy-1-(hydroxymethyl)ethyl]-5-[(2S)-2-hydroxypropanoylamino]-2,4,6-triiodoisophthalamide (C17H22I3N308; molecular weight, 777.09) represented by formula (I):
[0112]X-ray photography and CT scanning was carried out after one hour. The results are shown in FIG. 2. The X-ray images showed that the injected iopamidol was retained in the submucous tissue...
example 3
Retention of Physiologically Active Substances in the Submucous Tissue of Mouse Large Intestine
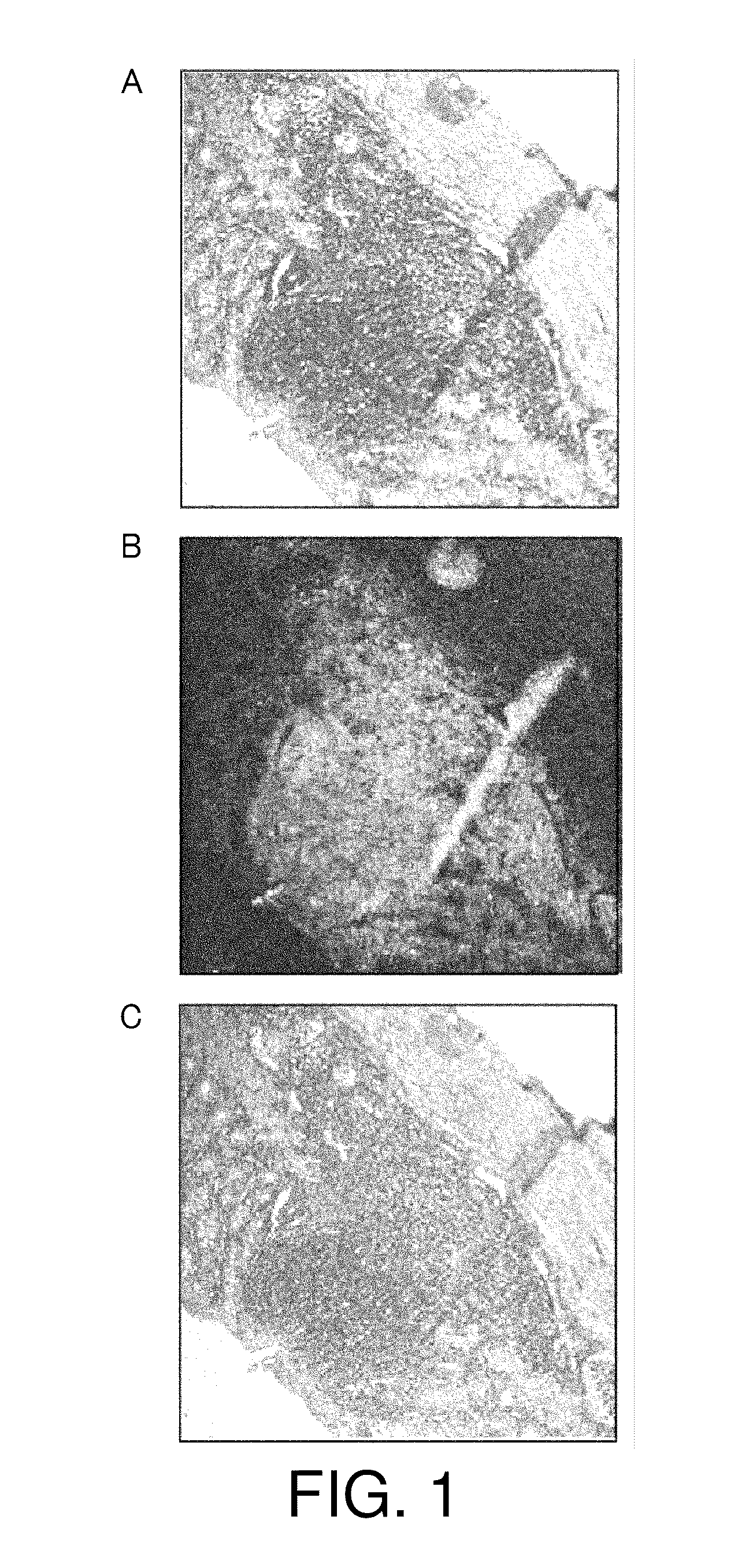
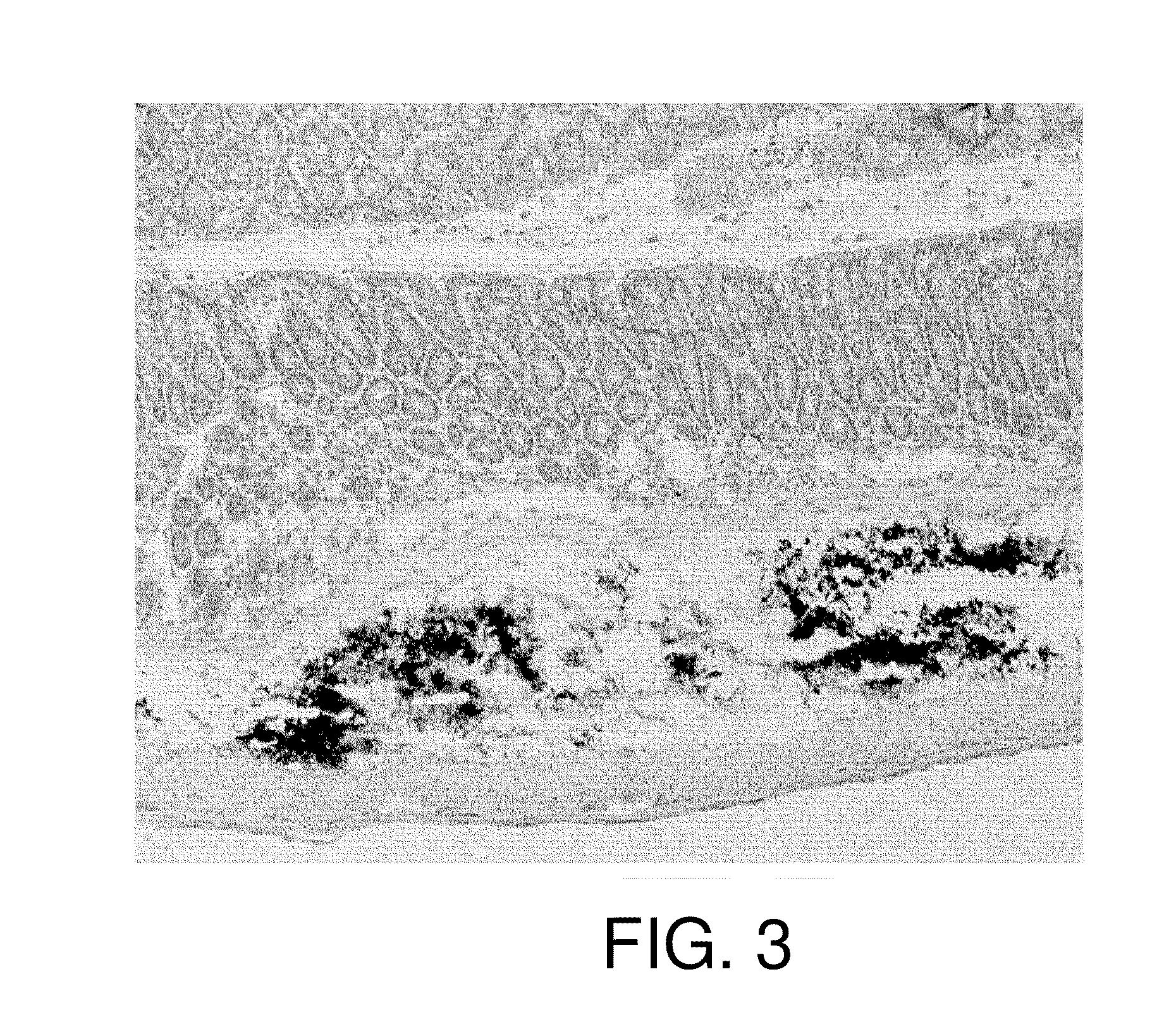
[0113]Next, the retention of physiologically active substances was assessed using mice, which are more common experimental animals. Eight-week-old female C57BL / 6J mice were anesthetized with Nembutal and laparotomized to expose the lower part of the large intestines. 20 μl of carbon particles (India ink), corresponding to a physiologically active substance of the present invention, alone was macroscopically injected to the submucous tissues. Then, the abdomen was closed.
[0114]Five days after, the mice were sacrificed to prepare tissue sections of the large intestine. The sections were observed under a light microscope. The result is shown in FIG. 3. The injected carbon particles were found to be retained within the submucous tissue without physical diffusion and leakage to other portions.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com