Administration of benzodiazepine compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
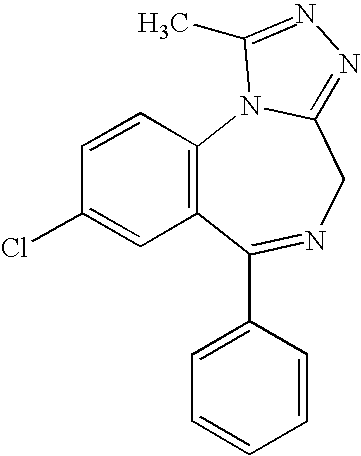
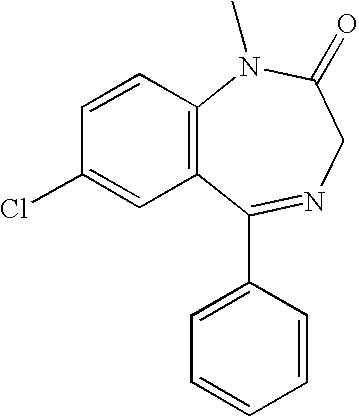
[0196]A pharmaceutical composition comprising diazepam is prepared. It is formulated as a solution to be delivered via a nasal delivery device. The composition is used to treat or prevent seizures associated with epilepsy in adults. Treatment is administered either before or after a seizure has begun. If the patient is seizing, it is administered as 1 puff from any nasal delivery device (1 puff at 5.0 mg / puff (5.0 mg / 0.1 mL and 0.1 mL / puff)) every 5 minutes until cessation of the seizure. However, it can be given as 1 puff per nostril in each nostril (2 puffs at 2.5 mg / puff (5.0 mg / 0.1 mL and 0.05 mL / puff)) every 5 minutes until cessation of the seizure. The composition according to this example is set forth in the following table.
TABLE 1-15.0 mg / 0.1 mLDiazepam70.0 mgα-tocopherol 0.1 mLethanol (qs ad to 0.1 mL)
example 2
[0197]A pharmaceutical composition comprising diazepam is prepared. It is formulated as a solution to be delivered via a nasal delivery device. The composition is used to treat or prevent seizures associated with epilepsy in children. Treatment is administered either before or after a seizure has begun. If the patient is seizing, it is administered as 1 puff from any nasal delivery device (1 puff at 2.0 mg / puff (2.0 mg / 0.1 mL and 0.1 mL / puff)). If the seizure fails to stop another dose may be administered after 5 minutes. However, it can be given as 1 puff per nostril in each nostril (2 puffs at 1.0 mg / puff (2.0 mg / 0.1 mL and 0.05 mL / puff)). If the seizure fails to stop another dose may be administered after 5 minutes. The composition according to this example is set forth in the following table.
TABLE 2-12.0 mg / 0.1 mLDiazepam70.0 mgα-tocopherol 0.1 mLethanol (qs ad to 0.1 mL)
example 3
Formulation of Diazepam Solutions
[0198]In general, benzodiazepine solutions may be formulated by combining one or more natural or synthetic tocopherols or tocotrienols and one or more lower alcohols or glycols and mixing until a homogeneous mixture is formed, adding the benzodiazepine drug to the homogeneous mixture, heating and mixing the ingredients until the benzodiazepine is fully dissolved in the homogeneous mixture, cooling the mixture, and bringing the mixture to its final mass or volume with lower alcohol or glycol.
[0199]Two different diazepam solutions were formulated by the foregoing process. Vitamin E USP and dehydrated ethanol USP were combined in the amounts set forth in the following table and mixed to form a homogeneous mixture. Diazepam in the amounts set forth in the following table was then added to the homogeneous mixture. The ingredients were heated to 40-45° C. with mixing until the diazepam was fully dissolved, thereby forming a solution. The solution was coole...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com