Methods for treating FSH related conditions with GnRH antagonists
a technology of gnrh and fsh, which is applied in the direction of drug compositions, extracellular fluid disorders, peptide/protein ingredients, etc., can solve the problems of lh no longer being produced, histamine-releasing activity, and worsening the condition, so as to and reduce the plasma fsh and lh levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
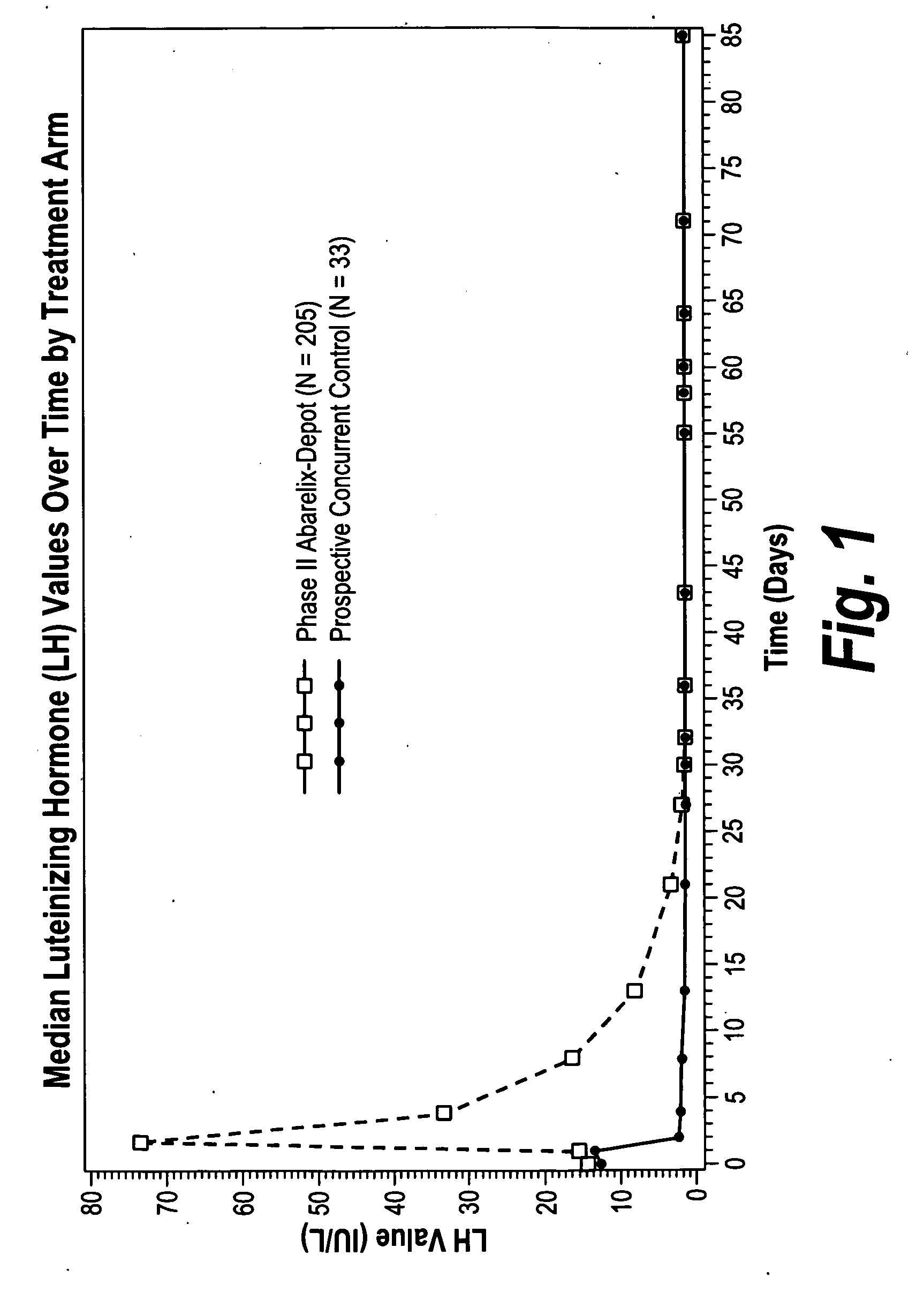
[0105]The following example describes the results of a multicenter, open-label Phase II study that enrolled 242 men (prospective concurrent control, N=33; abarelix depot, N=209). The groups were well matched for age, weight, height, race, body mass, stage of disease, and Gleason score (Table 4).
TABLE 4Demographic Characteristics Of Study Populationabarelix depotConcurrent controls(N = 209)(N = 33)Age (years)Mean (SD)72 ± 8.673 ± 6.2Range49-9359-84Weight (lbs)Mean (SD)188 ± 36.2185 ± 28.0Range104-315135-256No. (%) nonwhitea51 (24)11 (33)ECOG status (%)0201 (96) 33 (100)15 (2)023 (1)0Categories of disease (%)Stage D1 / D2b25 (12) 6 (18)Increasing PSA after53 (25) 8 (24)definitive local therapyNeoadjuvant / intermittent therapy131 (63) 19 (58)aAfrican American, Hispanic, Asian, othersbD1 = evidence of pelvic lymph note metastases; D2 = extrapelvic soft tissue or bone metastases
[0106]The institutional review boards of all participating institutions approved the study protocol, and all men ...
example 2
[0109]The following example describes results of experiments examining levels of FSH in plasma of human subjects treated with the GnRH antagonist abarelix, obtained from a Phase III clinical trial involving administration of abarelix to prostate cancer patients.
Study Design and Schema
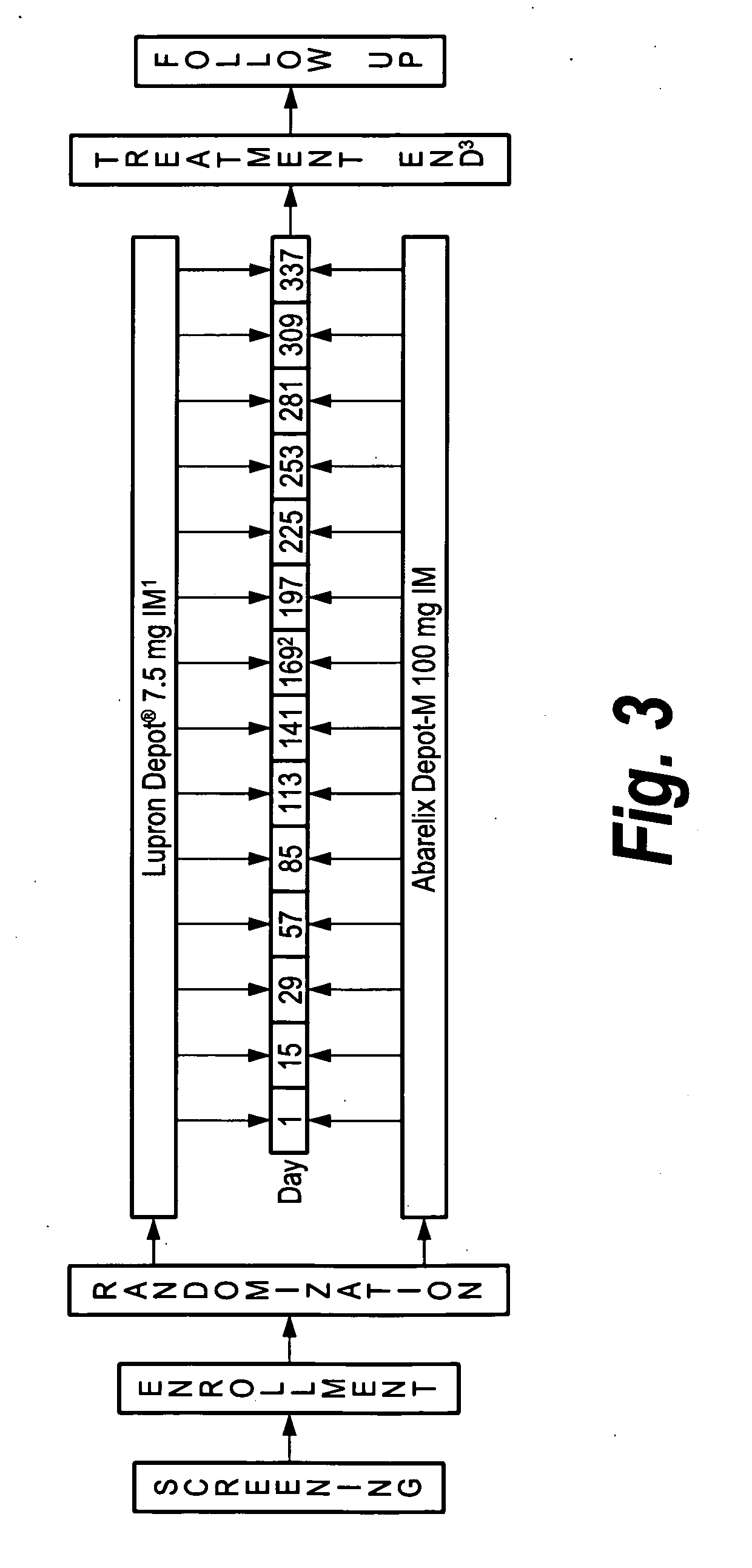
[0110]The following Phase III clinical studies A and B (using the abarelix depot) were blinded, randomized, parallel-group, multicenter phase 3 trials conducted in adult male patients with prostate cancer who were candidates for initial hormonal therapy, including patients with local or regional disease who were candidates for neoadjuvant hormonal therapy; patients with metastatic disease (stage D1 or D2); patients with rising prostate specific antigen (PSA) levels after radical prostatectomy, radiation therapy, or other local therapy; and patients scheduled for their initial course of intermittent therapy.
[0111]Patients were randomized to receive either abarelix depot 100 mg or active control medicatio...
example 3
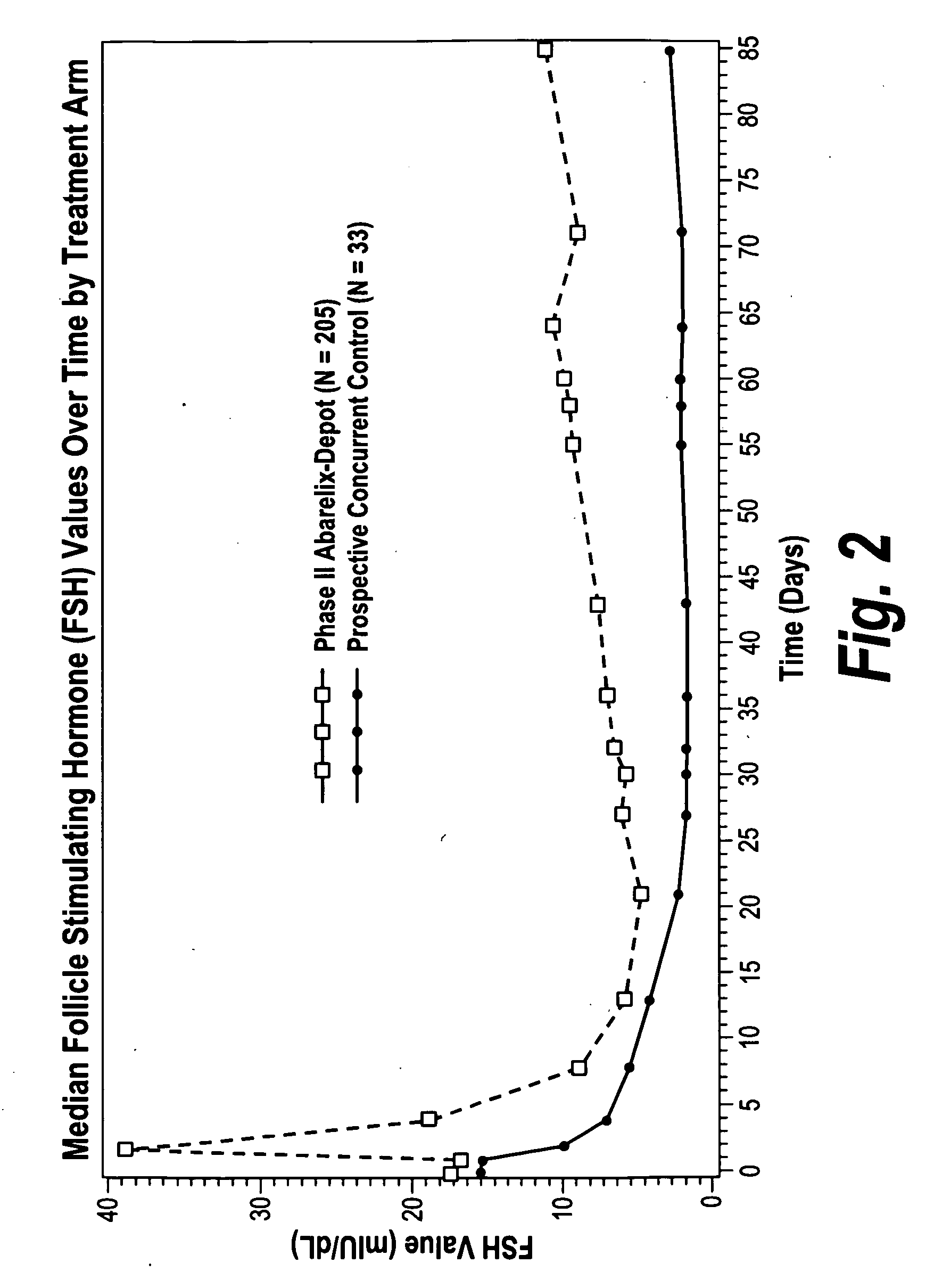
[0118]The effect of abarelix depot on FSH levels was further evaluated in phase III multicenter studies and compared to the effect of leuprolide±bicalutamide (L±B) on FSH levels. FSH levels were measured throughout the study in patients treated with injections of 100 mg abarelix depot and 7.5 mg leuprolide±oral daily bicalutamide 50 mg. Avoidance of FSH surge (50% over baseline FSH) on day 2 and maintenance of FSH suppression (≦FSH at baseline) on day 169 were evaluated in 512 patients. Data from the two studies was pooled for abarelix depot treated patients.
TABLE 5Patients (%) with FSH Responseabarelixleuprolide +depotleuprolidebicalutamideAvoidance of345 / 345 (100%)14 / 87 (16%) 8 / 80% (10%)FSH surgeMaintenance of312 / 322 (97%) 66 / 80 (82%)48 / 69 (70%)FSH Suppression(day 169)
[0119]FSH levels were rapidly suppressed with abarelix depot as compared to L±B. None of the abarelix depot treated patients versus 84% of the leuprolide (Lupron Depot®) treated and 90% of the L+B treated patients ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com