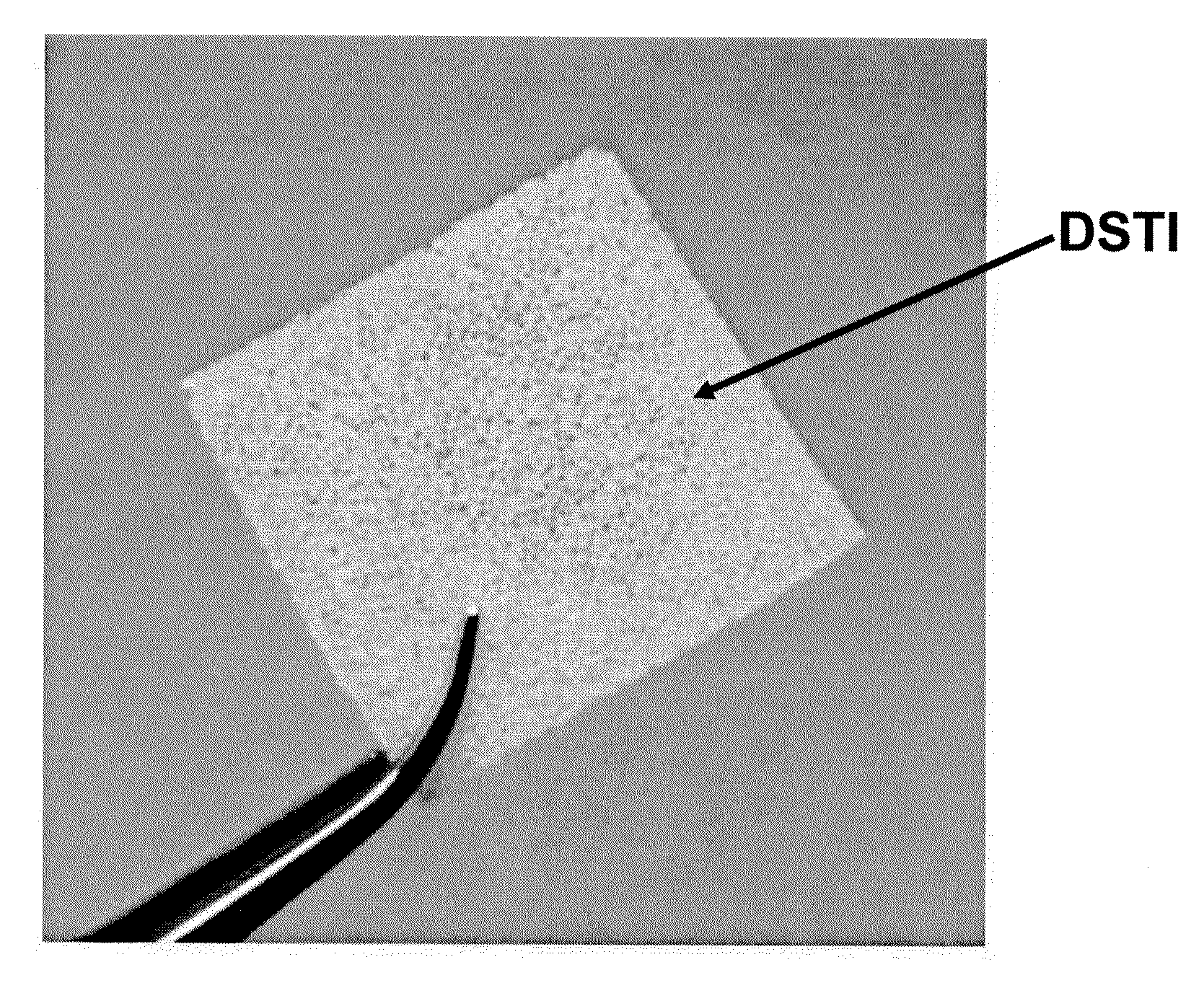
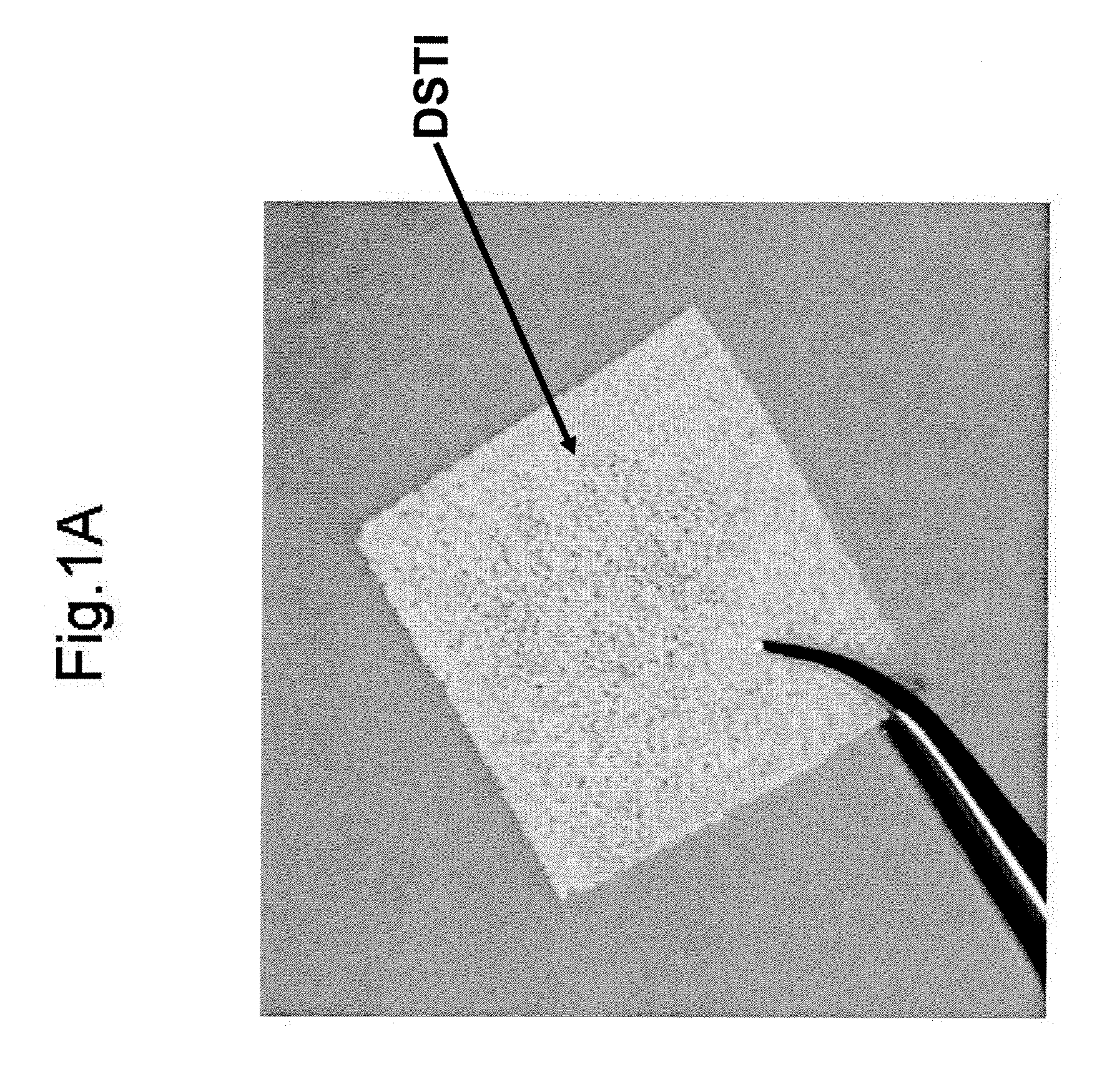
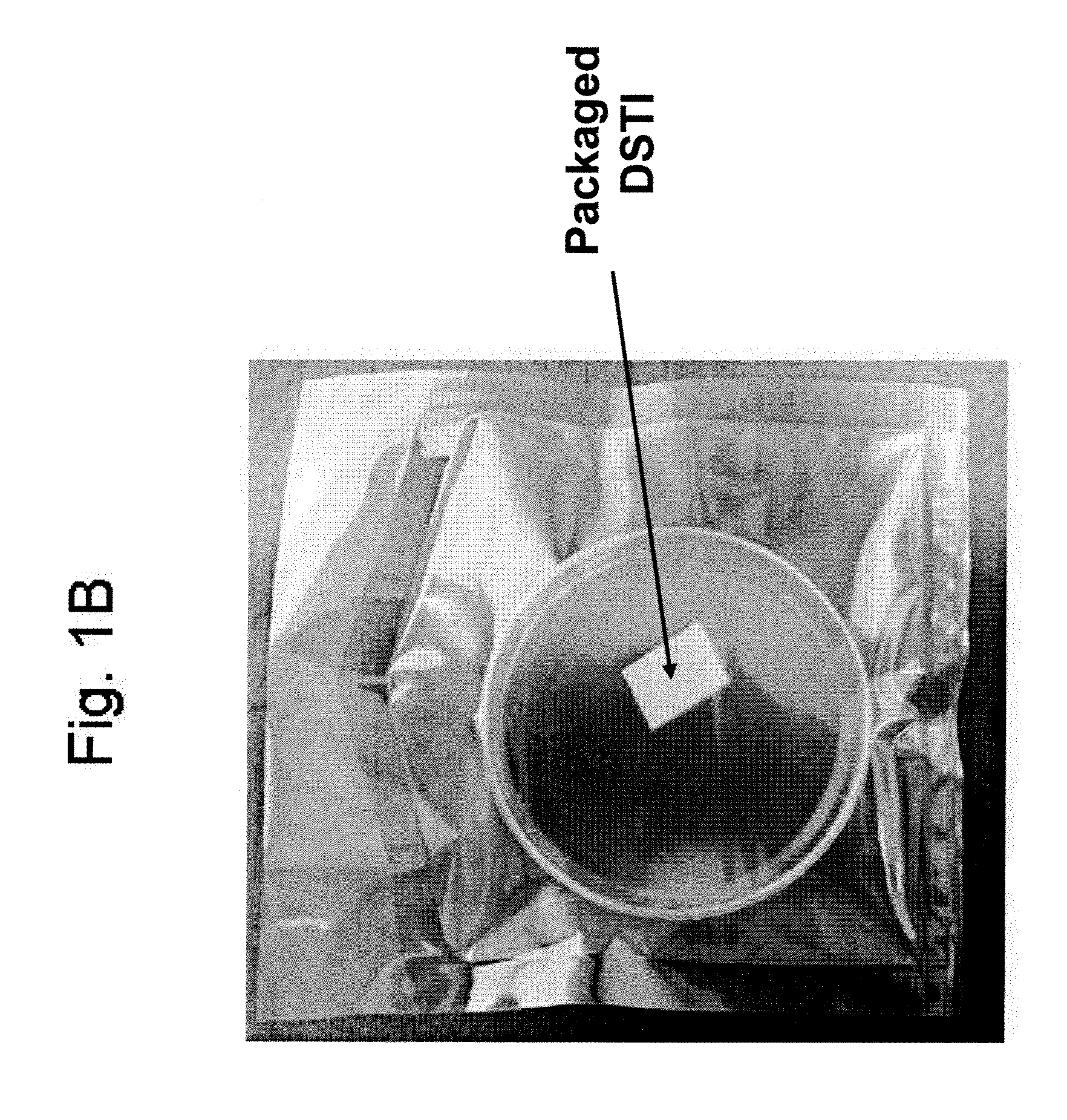
Method For Use Of A Double-Structured Tissue Implant For Treatment Of Tissue Defects
a tissue implant and double-structure technology, applied in the field of tissue implant use, can solve the problems of uneven and uncontrolled porosity, difficult handling of structures, and many of the above disclosed structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of the Primary Scaffold
[0252]This example describes one exemplary method for preparation of the collagen-based primary scaffold suitable as a structural support for preparation of the DSTI. Type I collagen is dissolved in a weak hydrochloric acid solution at pH 3.0 with the collagen concentration and osmolality of the solution adjusted to about 3.5 mg / ml and 20 mOsm / kg, respectively. The solution (70 ml) is poured into a 100 ml Petri dish and the Petri dish containing the collagen solution is centrifuged at 400×g for 30 minutes to remove air bubbles. Neutralization and subsequent precipitation or gelling is carried out in a 7 liter chamber containing 10 ml of 15% ammonia solution over a 45 minutes period. The precipitated collagen is then washed in a large excess of de-ionized water. The water is changed as many times as needed over next 3 days to remove formed salts and excess ammonia.
[0253]The solution is then subjected to unidirectional freezing over a period of about...
example 2
Preparation of a Basic Solution for a Secondary Scaffold
[0255]This example describes preparation of the Basic Solution used for formation of the secondary scaffold. The Basic Solution comprises a soluble collagen in admixture with a PLURONIC® surfactant. The Basic Solution is incorporated into the primary scaffold and processes into the double scaffold tissue implant or processed as a stand alone implant.
[0256]Solution for the secondary scaffold is prepared by mixing PLURONIC® F127 (2.32 mg, 0.29 mg / ml), obtained commercially from BASF, Germany, with 8 ml of a solution containing 2.9 mg / ml bovine type I collagen dissolved in hydrochloric acid (pH 2.0) at room temperature. The resulting solution is neutralized with 1 ml of 10× Dulbecco=s phosphate buffered saline (DPBS) and 1 ml of 0.1M NaOH to the final pH of 7.4.
[0257]In the alternative, the neutralization is achieved by ammonia aqueous solution or ammonia vapor in concentration sufficient to neutralize acid within the collagen sol...
example 3
Preparation of the Secondary Scaffold as Stand Alone Unit
[0258]This example illustrates preparation of the secondary scaffold as a stand alone implant or stand alone unit. For preparation of the stand alone secondary scaffold, the Basic Solution prepared in Example 3 is subjected to precipitation or gelling followed by dehydrothermal treatment.
[0259]The Basic Solution (2 ml) comprising collagen and PLURONIC® surfactant is placed in a small glass beaker and the beaker is placed into a chamber (approximately 9 liters) charged with 1% ammonia solution. The Basic Solution is allowed to precipitate in the chamber over a period of 15 minutes. The gelled or precipitated collagen is then washed in 500 ml of deionized water over a period of 30 minutes. The washing step is repeated three times. The washed gel or precipitate is placed on metal shelf of a freezer at −80° C. over a period of 30 minutes. The frozen gel or precipitate is removed from the freezer and lyophilized. Lyophilization is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com