Methods of Improving thermal transfer within a hydrocarbon reformig system
a hydrocarbon fuel and thermal transfer technology, applied in the field of hydrocarbon fuel reformation, can solve the problems of only being useful in intermittent hydrogen sources, not having the ability to follow large increases in load demand, and only having the ability to meet transient load demands. the effect of improving the ability of the hydrocarbon reforming process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
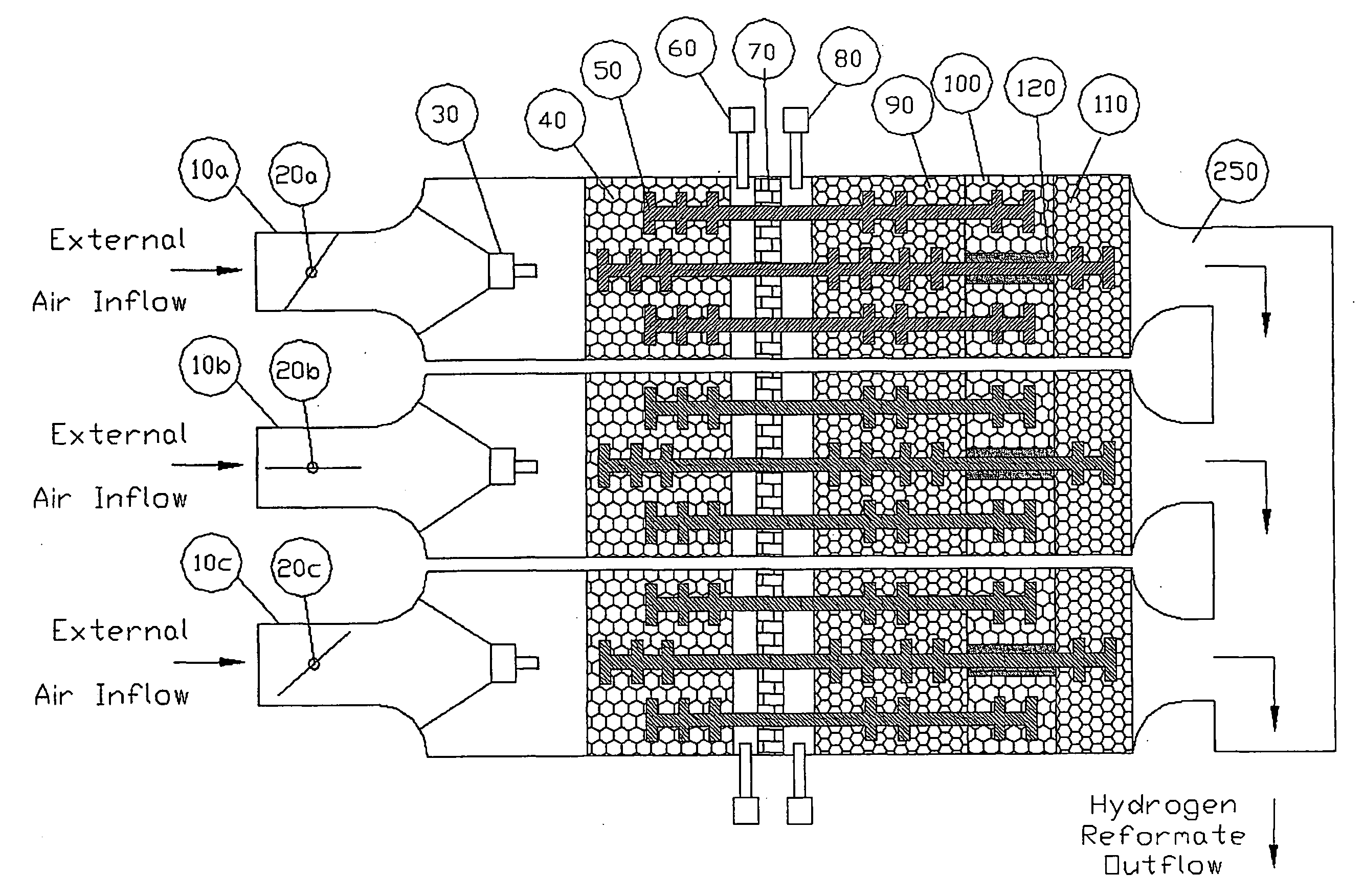
[0028]The ability of an air based reforming system to respond to any increase in hydrogen production demand is highly dependant upon that system's ability to provide appropriate thermal increases to the water feed stock, catalytic beds and other thermal masses of the containment components. The rate at which the thermal management system must respond is identical to the rate at which demand for hydrogen production changes. One system level approach to supplying more hydrogen for transient load increases, while the reformer's thermal management system struggles with maintaining the necessary process conditions, is to produce and store excess pressurized hydrogen in holding tanks.
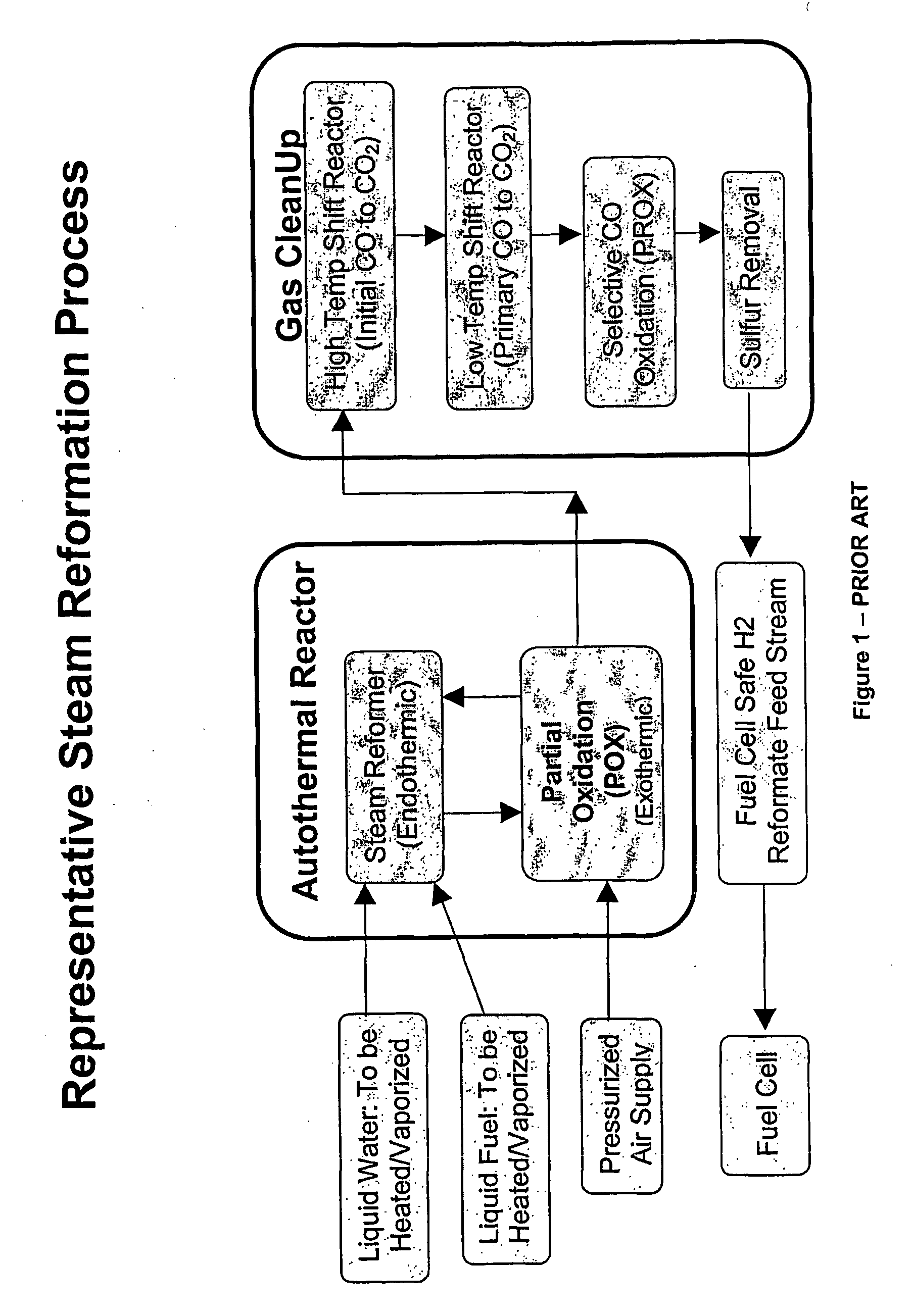
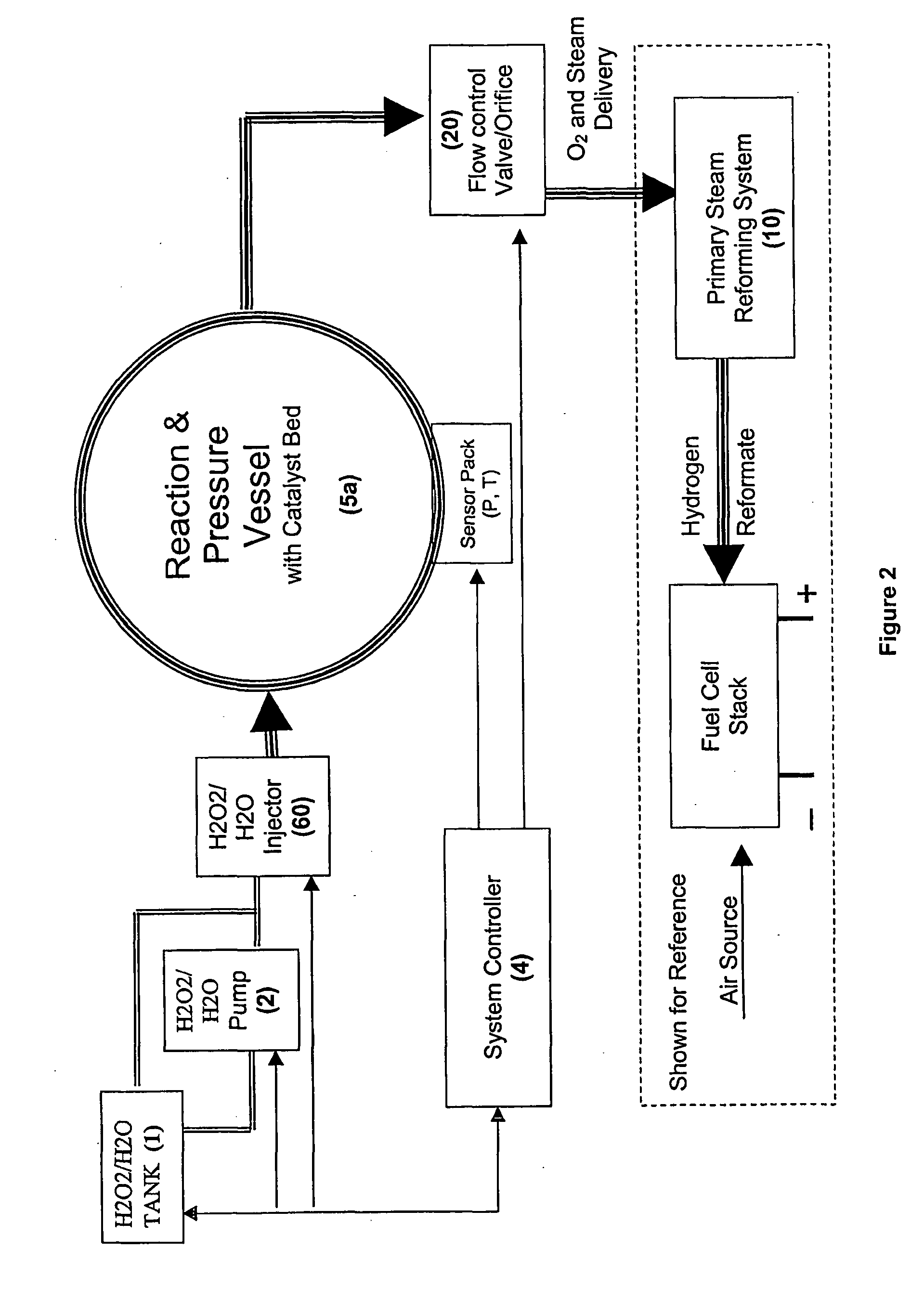
[0029]An alternative approach to obtaining supplemental hydrogen from a holding tank would be to introduce a supplemental, exothermic oxygen source at the beginning of the primary reforming process. To be effective, the oxygen source would ideally need to be free or substantially free of contaminates such as ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| chemical reaction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com