Circuit arrangement for injecting nucleic acids and other biologically active molecules into the nucleus of higher eucaryontic cells using electrical current
a technology of electrical current and nucleic acids, applied in the field of circuit arrangement, can solve the problems of low cell mortality, no circuit arrangement known so far, and no known circuit arrangemen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Transfection of Cytotoxic T Cells from Human Blood
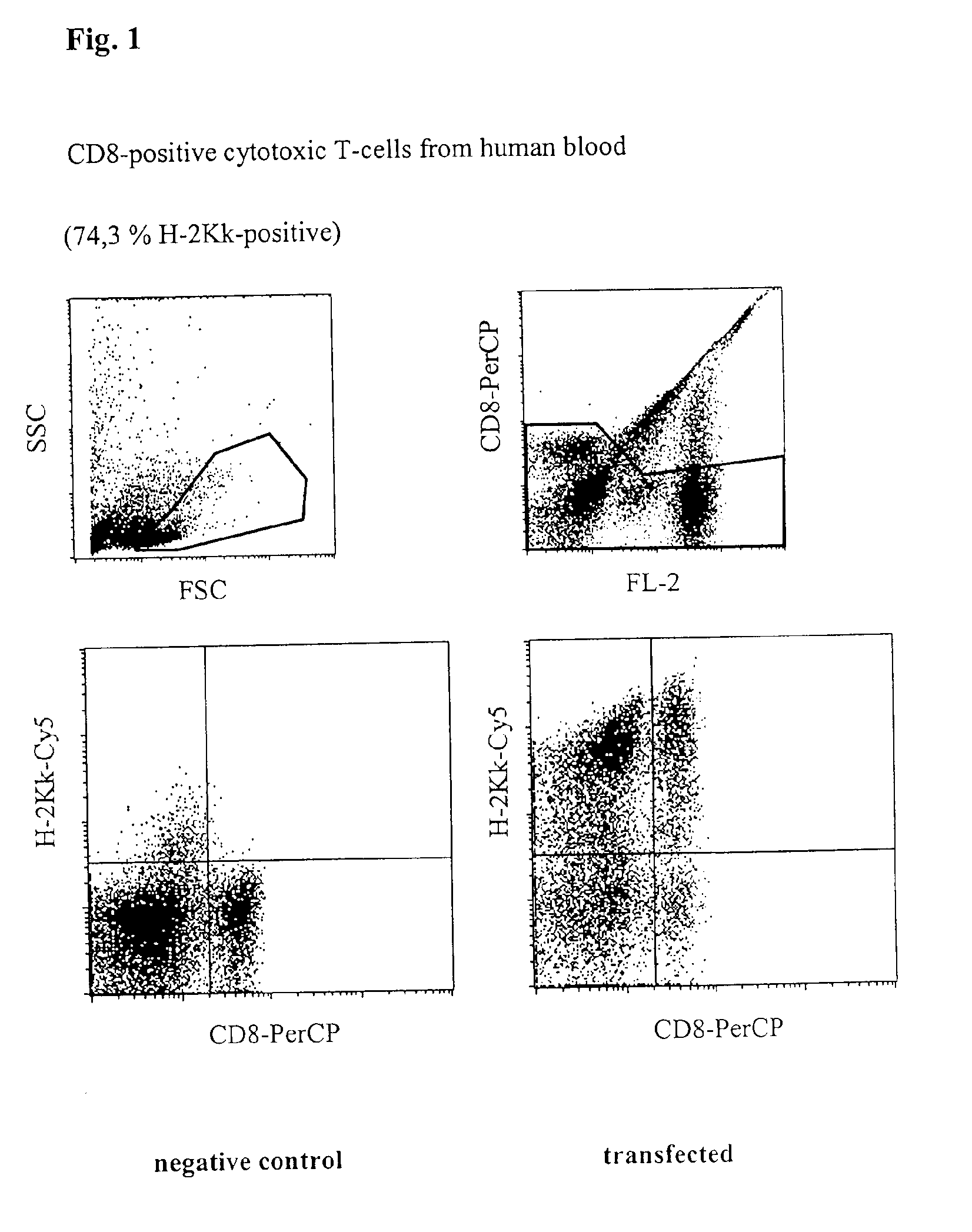
[0039]Freshly prepared unstimulated (non-dividing) mononuclear cells from peripheral human blood (PBMC) were transfected with a vector which codes for the heavy chain of the mouse MHC class 1 protein H-2Kk. 5×106 cells together with 5 μg of vector DNA in a buffer having a high buffer capacity (48 mM×pH−1) and high ionic strength (280 mM) were placed at room temperature in a cuvette having a 2 mm interelectrode gap and transfected by a 1000 V pulse of 100 μs duration, followed by a current flow having a current density of 5 A·cm−2 and 40 ms. Immediately afterwards, the cells were washed from the cuvette using 400 μl of culture medium, incubated for 10 minutes at 37° C. and then transferred to a culture dish with pre-heated medium. After incubating for 24 h, the cells were successively incubated with digoxigenin-coupled anti-H-2Kk-antibody and Cy5-coupled anti-digoxigenin-antibody, as well as with a PerCP-coupled anti-CD8-antibody to i...
example 2
Transfection of Human Haematopoeitic Stem Cells (CD34)
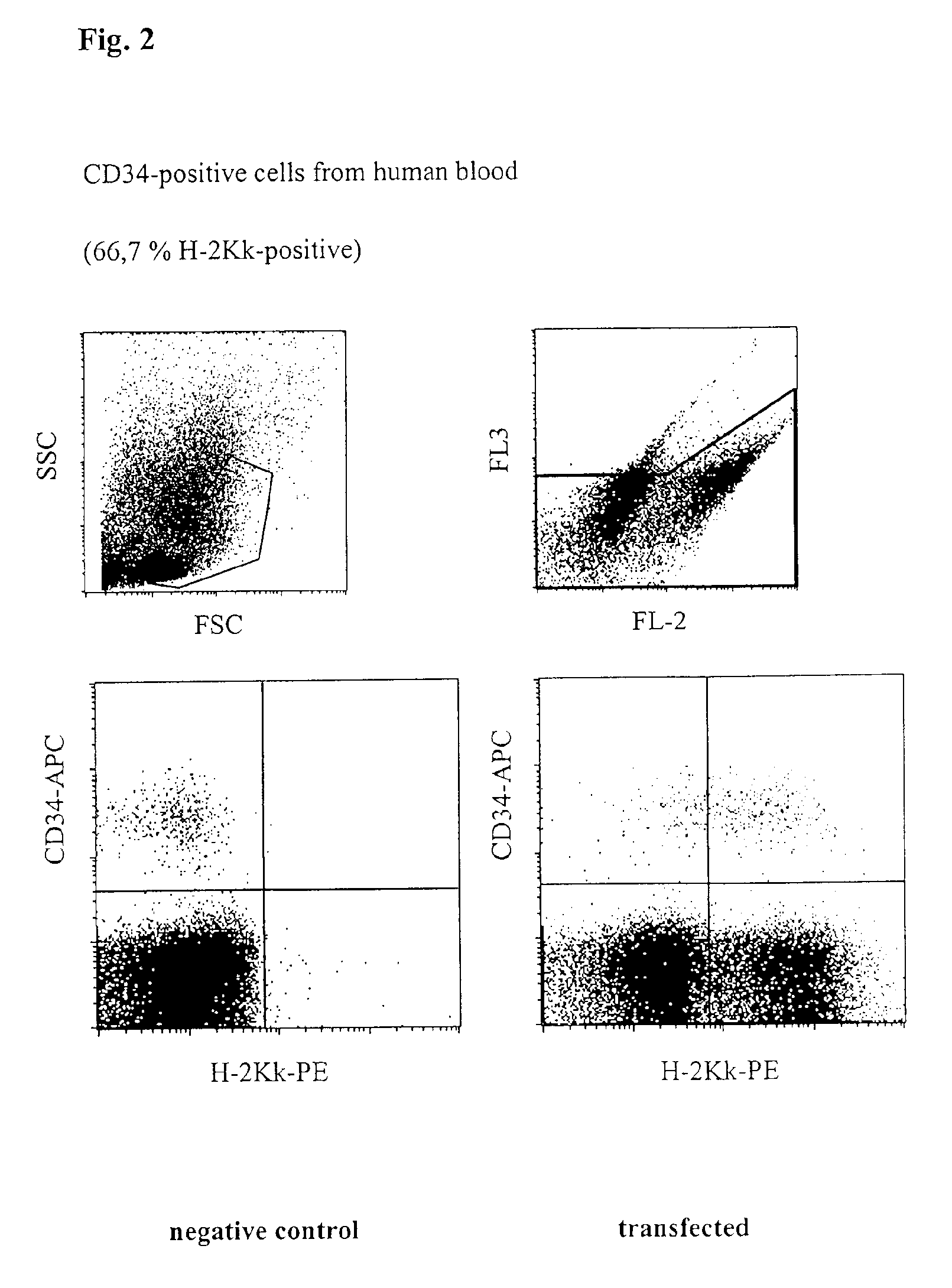
[0040]CD34-positive cells were pre-enriched from freshly prepared PBMC described as in Example 1 by magnetic cell sorting. Respectively 1×104 CD34-positive cells were then mixed with 1×106 PBMCs, placed together with 5 μg H-2Kk-expression vector DNA in a buffer having a high buffer capacity (54 mM×pH−1) and high ionic strength (260 mM) at room temperature in a cuvette having a 2 mm interelectrode gap and transfected by a 1000 V pulse of 100 μs duration, followed by a current flow having a current density of 4 A·cm−2 and 20 ms duration. Immediately afterwards, the cells were washed from the cuvette using 400 μl of culture medium, incubated for 10 minutes at 37° C. and then transferred to a culture dish with pre-heated medium. After incubating for 16 h, the cells were successively incubated with phycoerythrin-coupled anti-H-2Kk-antibody, as well as with an APC-coupled anti-CD34 antibody to identify human haematopoietic stem cells a...
example 3
Transfection of Human Neonatal Dermal Fibroblasts (NHDF-Neo)
[0041]Human neonatal dermal fibroblasts (5×105 cells) together with 5 μg H-2Kk-expression vector DNA were placed in a buffer having a high buffer capacity (67 mM×pH−1) and high ionic strength (380 mM) at room temperature in a cuvette having a 2 mm interelectrode gap and transfected by a 1000 V pulse of 100 μs duration, followed by a current flow having a current density of 6 A·cm−2 and of 33 ms duration. Immediately afterwards, the cells were washed from the cuvette using 400 μl of culture medium, incubated for 10 minutes at 37° C. and then transferred to a culture dish with pre-heated medium. After incubating for 5 h, the cells were incubated with a Cy5-coupled anti-H-2Kk-antibody and analysed using a flow cytometer (FACScalibur, Becton Dickinson). The number of dead cells was determined by staining with propidium iodide. As shown in FIG. 3, 93% of the cells express the H-2Kk antigen which corresponds to a very high transf...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current density | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com