Pulsatile release histamine H2 antagonist dosage form
a pulsatile release and histamine technology, applied in the field of pulsatile release histamine h2 antagonist dosage form, can solve the problems of limited size or material, few orally applicable pulsatile release systems, and inability to maintain a constant blood level of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0089]Nizatidine (5787.7 g) was slowly added to an aqueous solution of hydroxypropylcellulose such as KLUCEL LF (hydroxypropyl cellulose) (643.1 g) and mixed well. #25-30 mesh sugar spheres (3700 g) were coated with the drug suspension in a Glatt fluid bed coater. The drug containing particles were dried, and a seal coat of Opadry Clear (2% w / w) was first applied. These drug containing IR Beads were provided with an outer membrane by spraying a solution of 1:1 blend of ethylcellulose and HPMCP plasticized with diethyl phthalate in 98 / 2 acetone / water in a fluid bed coater for a weight gain of approximately 39-40%. The coated particles are cured at 60° C. until the polymers were coalesced to produce TPR Beads. Pulsatile Release Nizatidine Capsules, 150 mg, were manufactured by filling 75 mg IR Beads and 75 mg TPR Beads into size 0 hard gelatin capsules using a MG Futura capsule filling equipment. The drug release testing was performed using USP Apparatus 2 (Paddles @ 50 rpm) in 0.1N H...
example 2
[0090]Nizatidine (168 kg) was slowly added to an aqueous solution of hydroxypropylcellulose such as KLUCEL LF (hydroxypropyl cellulose) (18.6 kg) and mixed well. #25-30 mesh sugar spheres (107.4 kg) were coated with the drug suspension in a Glatt fluid bed coater, equipped with a 32″ bottom spray Wurster insert. The drug containing particles were dried, and a seal coat of Opadry Clear (2% w / w) was first applied and dried in the Glatt fluid bed unit as a precautionary measure to drive off excessive surface moisture. These drug containing IR Beads were provided with an outer membrane by spraying a solution of 1:1 blend of ethylcellulose and HPMCP plasticized with diethyl phthalate in 98 / 2 acetone / water in a fluid bed coater for a weight gain of approximately 39-40%. The coated particles are cured at 60° C. for 4 hours to produce TPR Beads (batch size: 300 kg). Pulsatile Release Nizatidine Capsules, 150 mg, were manufactured by filling 75 mg IR Beads and 75 mg TPR beads into size 0 har...
example 3
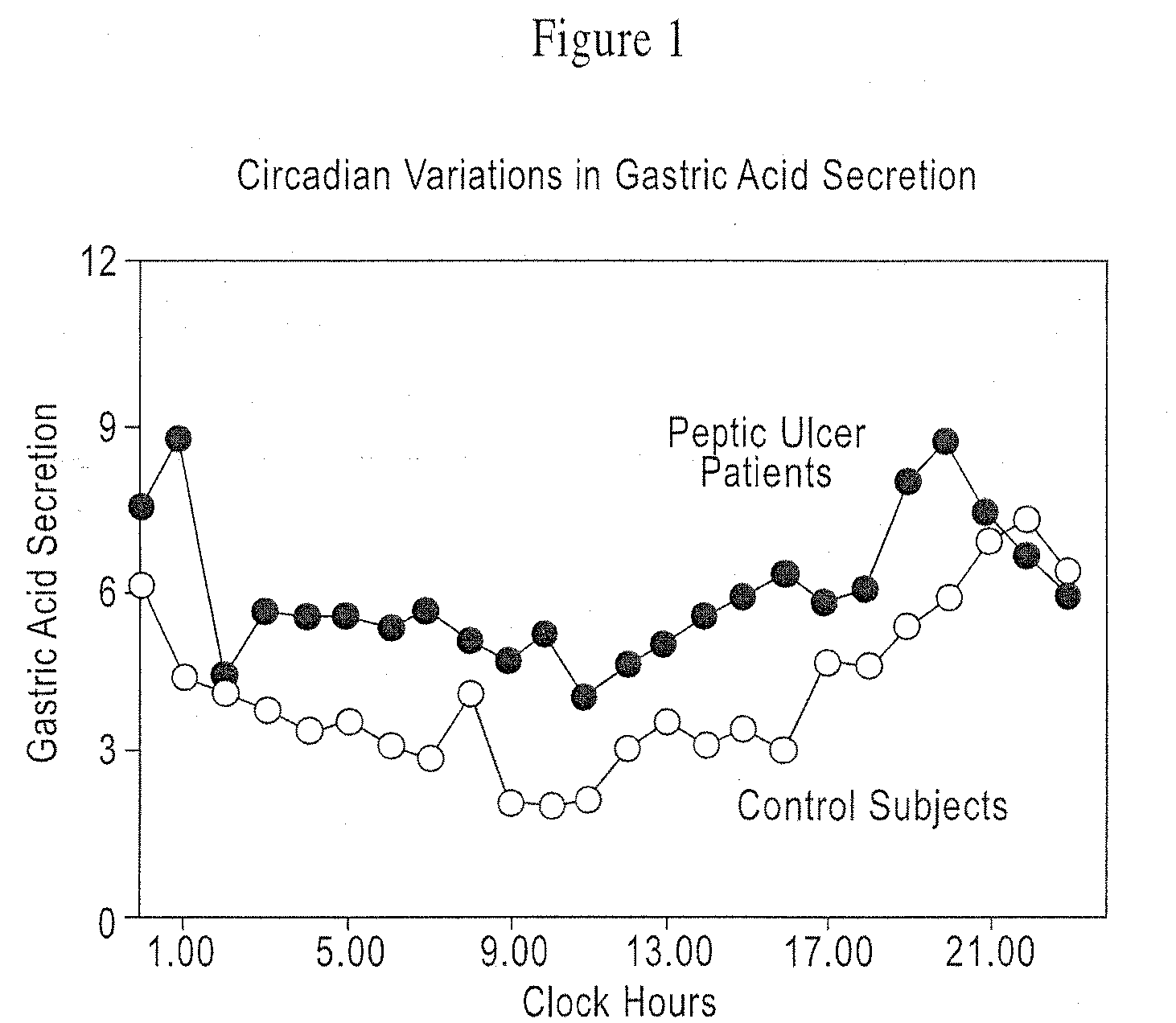
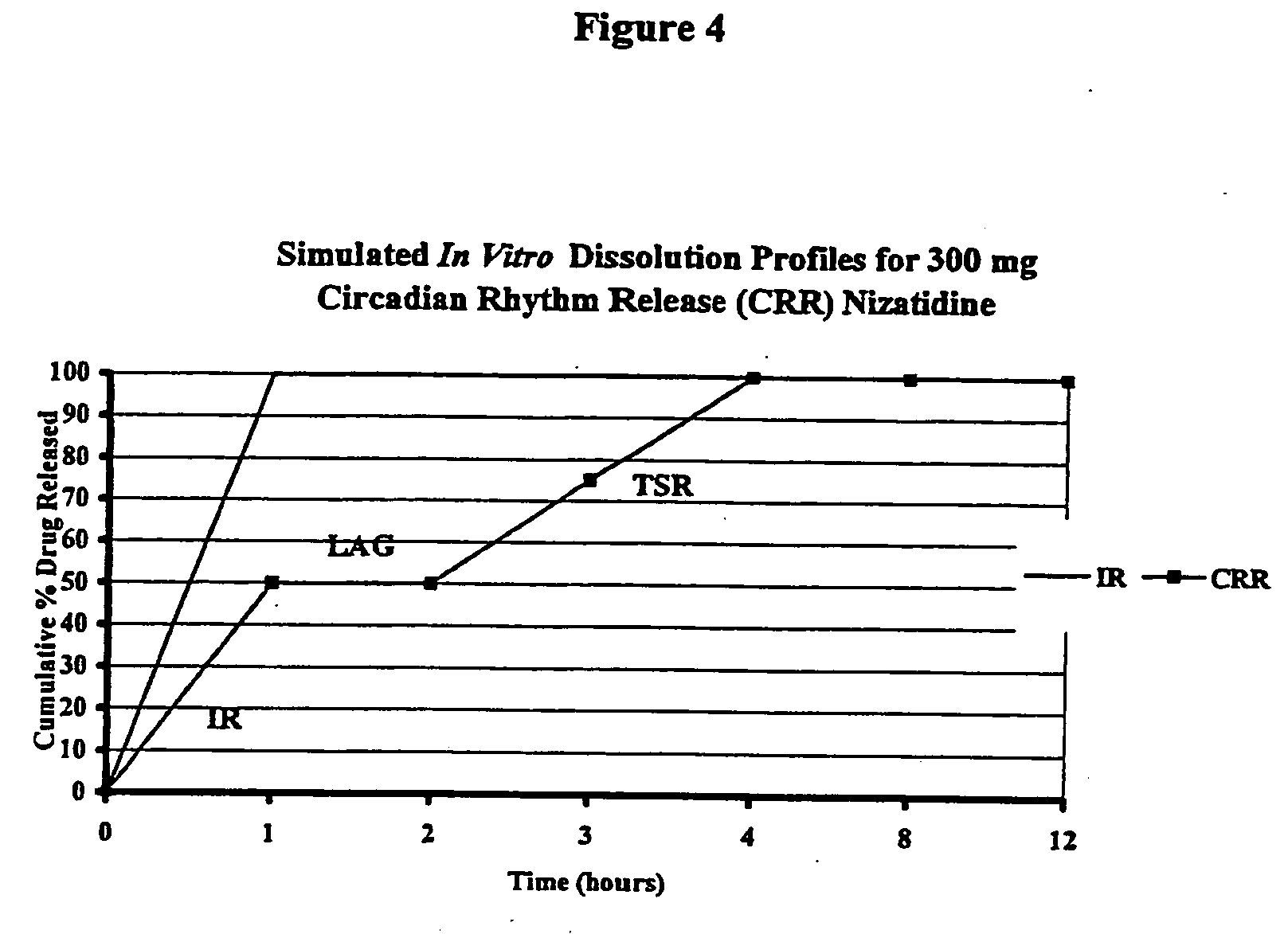
[0091]In order to assess the type of in vitro release profile needed to achieve a circadian rhythm effect under in vivo conditions, a modeling exercise was performed using the pharmacokinetic parameters for nizatidine. A diurnal variation in the pharmaco-kinetics of nizatidine has been reported by Jamali, A. et al., Journal of Clinical Pharmacology 35: 1071-1075 (1995), is incorporated in its entirety). A pharmaco-kinetic modeling was done separately to try to mimic both evening and day time results individually. Mean serum concentrations of nizatidine achieved in healthy volunteers were taken from the same literature. Theoretical in vitro dissolution profile (FIG. 4) as well as in vivo serum levels achieved during evening and daytime dosing, were simulated using the pharmaco-kinetic models developed. The advantages of a pulsatile dosage form are evident in attached FIG. 5 that compares simulated serum levels achieved with an immediate release dose of nizatidine versus the proposed ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com