Methods for detecting pathological sites
a pathological site and detection method technology, applied in the field of site directed therapy, can solve the problems of not being able to provide a large amount of cytotoxic compounds without hampering the targeting specificity of known targeting moieties, antibodies, and often not being able to provide isotopes with a suitable half-life, and isotopes with a long half-life cannot be chosen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
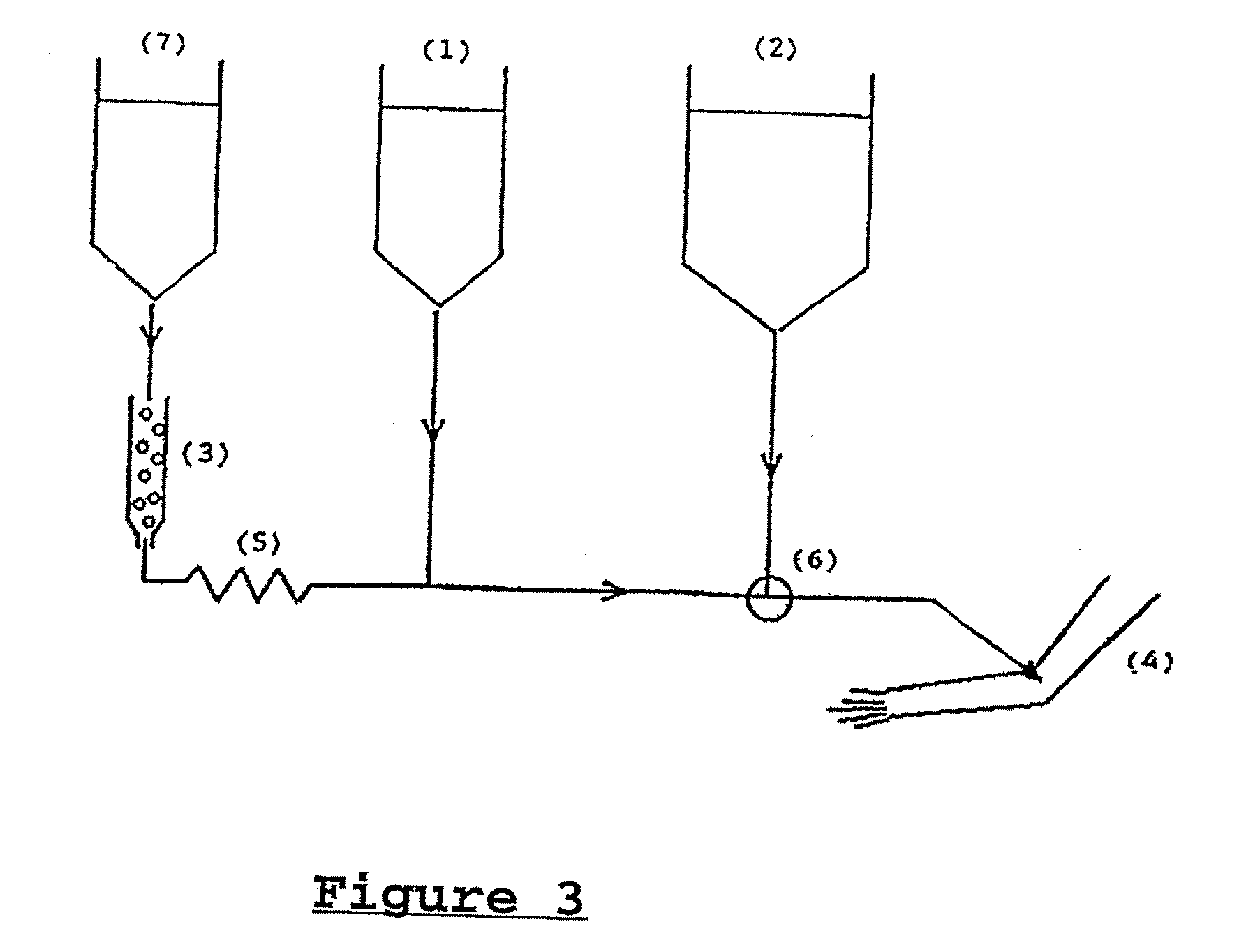
[0111]The separation chemistry of the various radioactive elements mentioned in the text before has been sorted out decades-ago and is well-documented in the public literature. Examples are references (3) and (4). 225Ac can be separated from 229Th on a Dowex 50 ion exchanger by stripping with 4N HNO3. After evaporation of the acid, the 225Ac can be dissolved again in 0.5N HNO3 in a fixed concentration and absorbed in the appropriate amount on Dowex 50, which then becomes the material in the mini-column (3) of FIG. 3.
example 2
[0112]0.68±0.07 mCi of 225Ac was obtained from the European Joint Research Centre. This was loaded on a MP-50 cation exchange resin (Bio-Rad). The formed 213Bi was eluted with a mixture of 50:50 10% NH4Ac:MeOH with a pH of 6.75. An autoburet was used to deliver 35 μl of eluant per minute; alternatively, manual elution was done at 50 μl amounts of eluant per minute.
[0113]In a few experiments, it was necessary to purify the 213Bi. This was accomplished by heating the eluant to dryness in a 10 ml beaker containing 0.5 ml of conc. HNO3. After evaporation under an IR lamp, the bismuth activity was transferred to a column of MP-50 resin (2×30 cm, pre-equilbrated with 0.1M HNO3). The resin was washed with 0.2 ml H2O. Then the 213Bi was eluted with 0.5 ml of Hcl and HI. Various concentrations of Hcl and HI have been tried. FIG. 4 shows the elution patterns for 213Bi. In all cases, the elution is rapid and quantitative. All of the isotope can be obtained within 5 to 10 minutes after the star...
example 3
[0115]Radiolabeling was done by adding enough 3M NH4Ac to the 213Bi stock to achieve pH 4.0-5.0. Then 53 μl or 106 μl of a 4.7 mg / ml solution of monoclonal antibody B3 coupled with the chelator CHX-DTPA (cyclohexyldiethylenetriaminepenta acetic acid) according to the method described in (5) were gently mixed into the solution. After a fifteen minute reaction time, 1.5 μl of 0.1M EDTA were added. The solution was transferred to a 1 ml syringe with 0.2 ml wash. The solution was then injected into the HPLC (high pressure liquid chromatography) having a TSK 3000 column. The buffer was 0.02 M MES / Cl− (MES=morpholino ethane sulfonic acid), 0.15 M NaCl, pH 6.5. Elution of the B3 antibody occurred at 7.5 minutes. The amount of 213Bi incorporated into the antibody was monitored with an in-line radiochemical detector (Beckman). All activity measurements of 213Bi were corrected for decay (t1 / 2=45.6 min). Results are depicted in Table 2. Activities of 225Ac, 221Fr or 217At were not detectable i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| extracellular structure | aaaaa | aaaaa |
| half life | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com