Anode for use in aluminum producing electrolytic cell
an electrolytic cell and anode technology, applied in the field of anodes, can solve the problem of unfavorable metal distribution on complex shaped workpieces
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
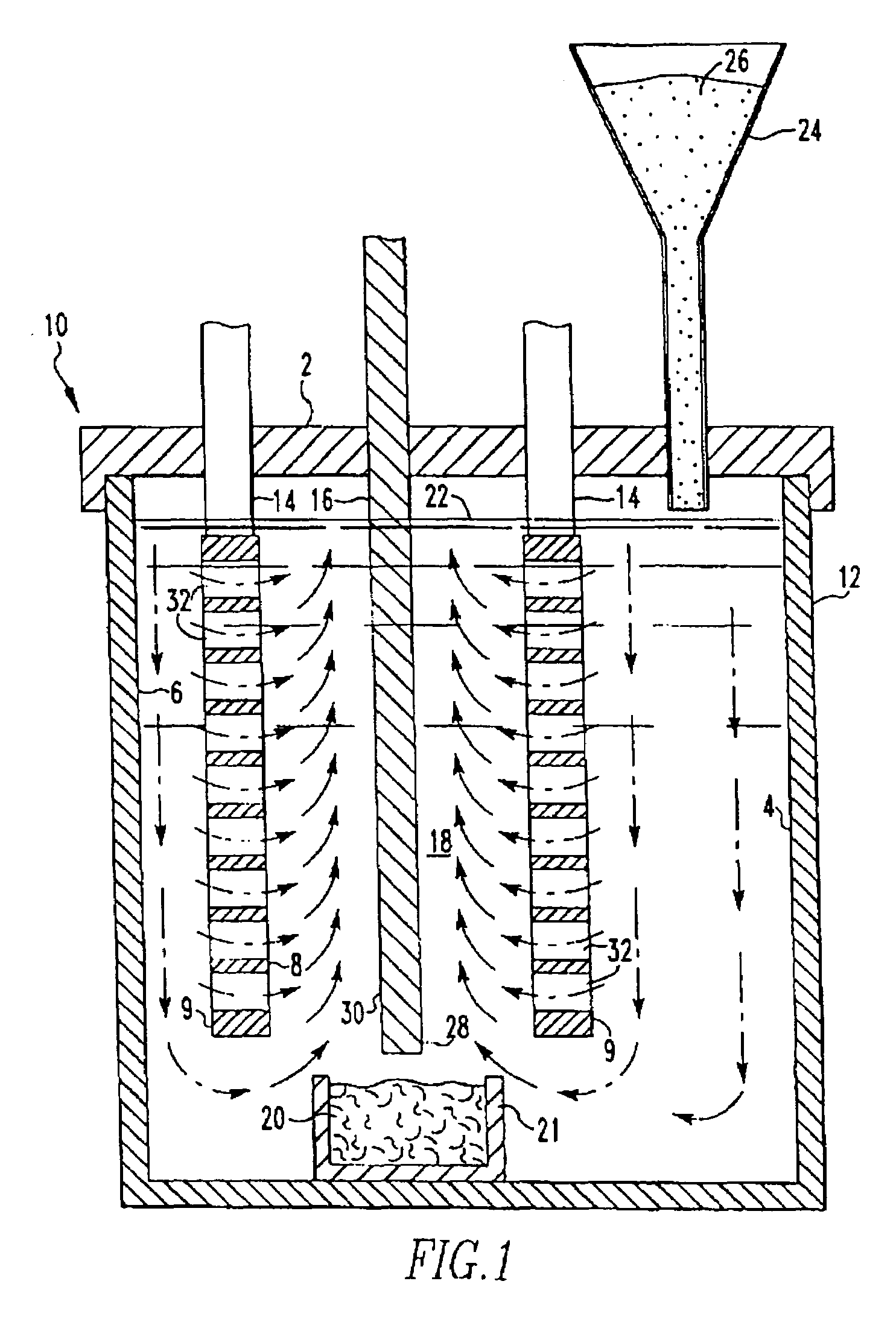
[0058]This invention was tested in a 150 A cell having the configuration shown in FIG. 1 with alumina added to the cell substantially continuously. The cell comprised an alumina ceramic container. Within the ceramic container was placed a vertical cathode suspended through the lid of the container and connected to a bus bar. On either side of the cathode, two anodes were positioned or suspended through the lid and connected to bus bar. The anodes were 4 inches by 5 inches by ⅜ inch thick. Each anode was drilled to provide 112 holes ¼ inch in diameter. The anodes were comprised of 18 wt. % Cu, 20 wt. % Ni, 2 wt. % Sn and 60 wt. % Fe, and the cathode was TiB2. The cell contained a molten salt bath comprised of 37.93 wt. % sodium fluoride and 62.07 wt. % aluminum fluoride. The top of the cell was sealed with an insulating lid and the cell was maintained at an operating temperature of 770° to 780° C. which was above the melting point of the salt bath and the aluminum metal. The alumina ...
example 2
[0059]A second test was run for 503 hours. The composition of the anode was the same as in Example 1. The operating temperature was 800° C., the current density was 0.53 amps / cm2, and the ACD was one inch. The average voltage for the entire run was 4.08 volts and was steady at the completion of the run. Smelter grade alumina was used. The melt purity was 99.83% for the entire test. This included impurities from the alumina.
[0060]The use of the new anode results in reduced voltage due to reduced surface oxide layer resistance compared to anodes using only Cu—Ni—Fe. Further, the new anode permits increased usage of lower cost iron as the primary alloying component. Also, compared to a Cu—Ni—Fe anode, the use of Cu—Ni—Fe—Sn results in long term stabililty of the surface oxide layer. In addition, the new Cu—Ni—Fe—Sn anode results in better metal purity. Furthermore, the Cu—Ni—Fe—Sn anode results in improved corrosion resistance at the cell bath interface, eliminating the need for extra ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com