4c
a technology of 4c and primers, applied in the field of 4c, can solve the problems of requiring a substantial amount of prior, and achieve the effect of increasing the scale and resolution of analysis, and reducing the cost of so many primers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials & Methods Section that goes with FIGS. 2, 13, 14, 15, 16,17, 19.
4C Technology
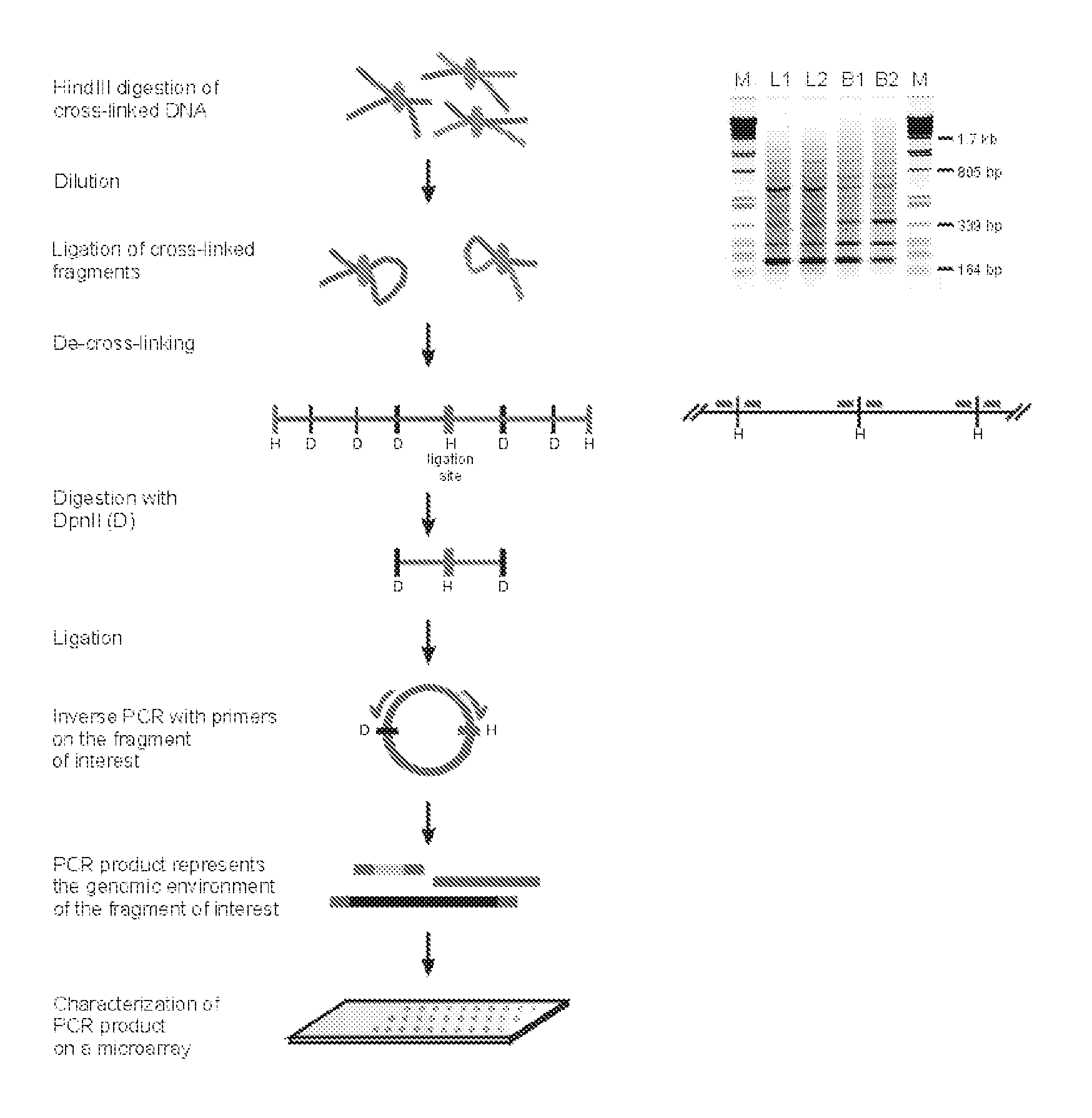
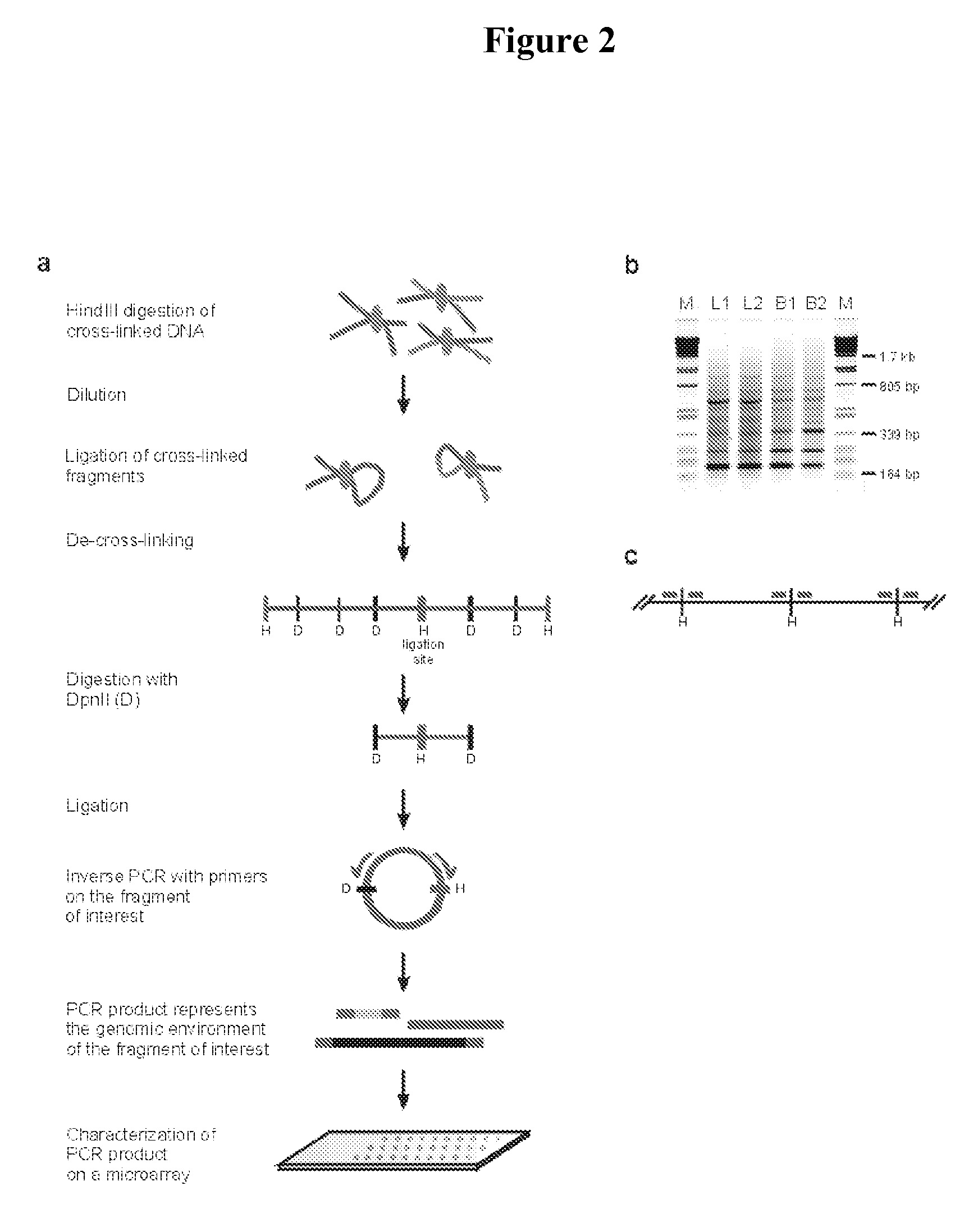
[0524] The initial steps of the 3C technology procedure were performed as described previously (Splinter et al. (2004). Methods Enzymol 375, 493-507 (2004), yielding ligation products between HindIII fragments. This HindIII-ligated 3C template (˜50 μg) was digested overnight at 100 ng / μl with 50 U of a secondary, frequent cutting, restriction enzyme, being either DpnII (HS2, Rad23A) or NlaIII (1-major). To avoid constraints in DNA circle formation (Rippe et al. (1995) Trends Biochem Sci 20, 500-6), care was taken to choose a secondary restriction enzyme that did not cut within about 350-400 bp from the HindIII restriction site that demarcates the restriction fragment of interest (i.e. the ‘bait’). After secondary restriction enzyme digestion, DNA was phenol extracted, ethanol precipitated and subsequently ligated at low concentration (50 μg sample in 14 ml using 200 U ligase (Roche), 4 hours a...
example 2
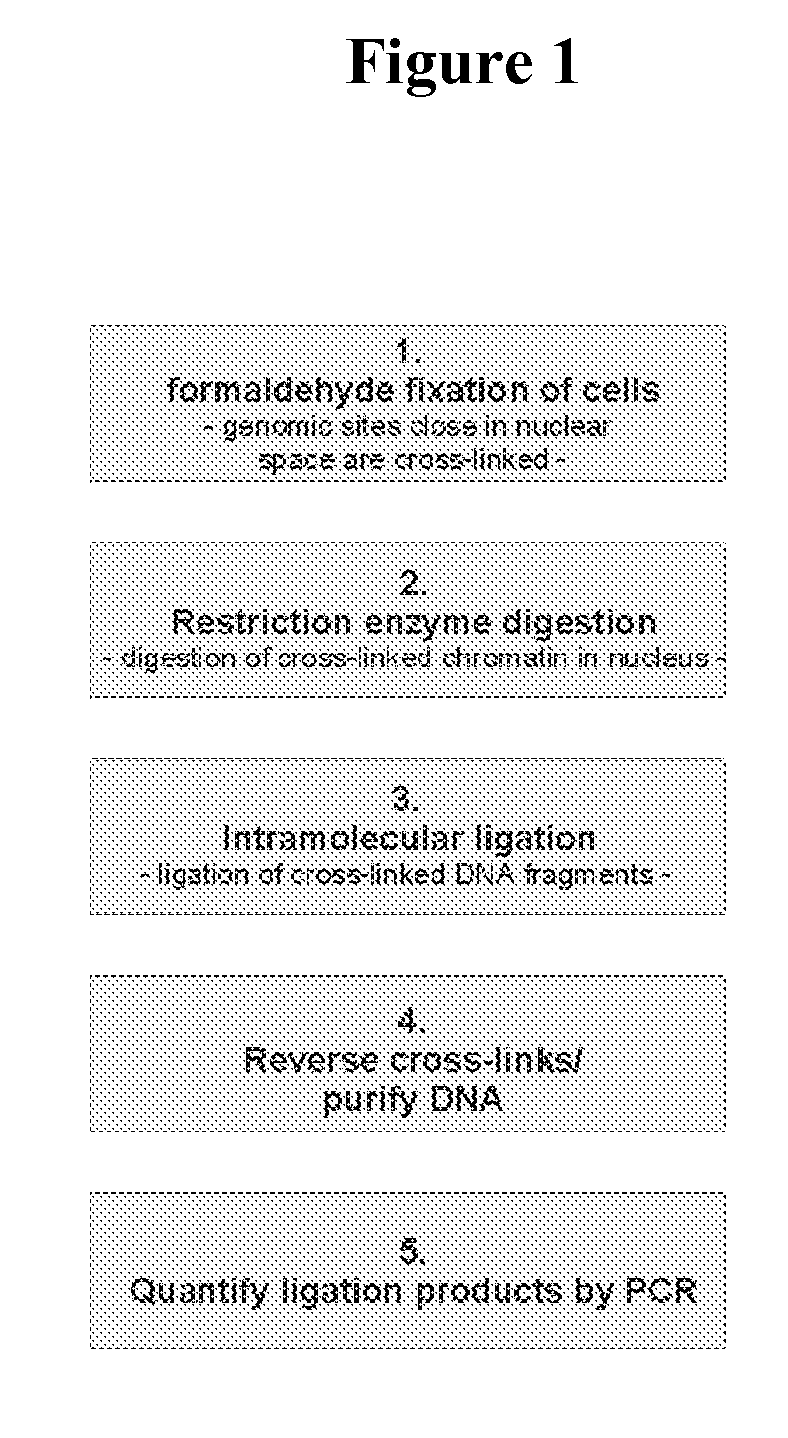
[0535] The 3C procedure (i.e. formaldehyde fixation, (primary) restriction enzyme digestion, re-ligation of cross-linked DNA fragments and DNA purification) is carried out essentially as described (Splinter et al., (2004) Methods Enzymol. 375: 493-507), yielding a DNA mixture (‘3C template’) containing restriction fragments that are ligated because they were originally close in the nuclear space.
[0536] Inverse PCR is performed to amplify all fragments ligated to a given restriction fragment (‘bait’; chosen because it contains a promoter, enhancer, insulator, matrix attachment region, origin of replication or any other first (target) nucleotide sequence).
[0537] For this, DNA circles are created by digesting the 3C template with a secondary restriction enzyme (preferably a frequent cutter recognizing tetra- or penta-nucleotide sequences), followed by ligation under dilute conditions such that intra-molecular interactions are favoured. To minimise a bias in circle formation due to to...
example 3
Detection of Translocation Using 4C Technology
[0551] 4C technology is used to measure the interaction frequencies for a given sequence X present on a given chromosome A in cells from a healthy subject and in cells from a patient carrying a single, reciprocal, translocation between chromosome A and B with the breakpoint being close to sequence X (as shown in FIG. 8).
[0552] In normal cells this analysis reveals elevated hybridization signals (i.e. frequent interactions with X) for (almost) every probe located within 0.2-10 Mb of sequence X on chromosome A (the actual size of the chromosomal region showing strong cross-linking signals depends mostly on the complexity of the sample that was hybridized to the array). Elsewhere on the same chromosome A, as well as on other chromosomes, no such large region (on the linear DNA template) of probes with elevated hybridization signals is observed.
[0553] In patient cells however, hybridization signals with all chromosome A probes located on...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| sizes | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com