Electrolytic generation of nitrogen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
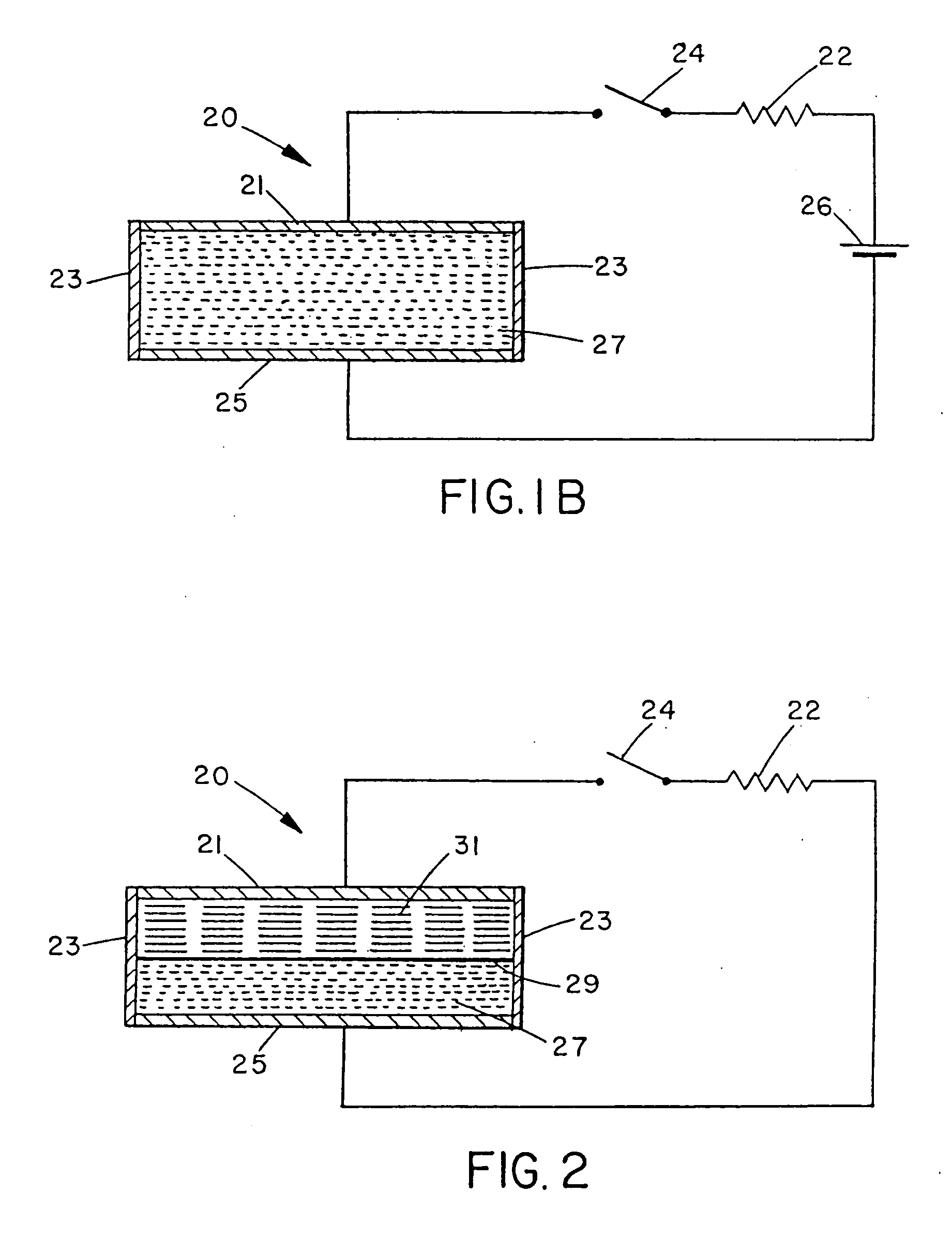
[0035] In one embodiment, a nitrogen gas generator is assembled as shown in FIG. 1B, comprising: [0036] (a) a circuit comprising an external energy source 26, such as two 1.5 V alkaline batteries connected in series; a resistor 22, such as a variable resistor from 1 to 100 kOhm; and a switch 24; [0037] (b) an undivided electrochemical cell 20 comprising: [0038] i) electrolyte solution 27, comprising an active nitrogen compound, in one embodiment, methyl hydrazine carboxylate (about 0.1 to 4M), urea (about 0.1 to 1M), ammonium sulphate (about 0.1 to 2M) and water, all absorbed in a cellulose sponge; [0039] ii) anode 21 and cathode 25, which in various embodiments may be graphite fibre impregnated with a polymer such as Nylon™ or polypropylene GRAFOIL, pyrolytic carbon, carbon black, platinum or gold.
[0040] The probable (but unknown) methyl hydrazine carboxylate anode reaction (as in examples 6, 8 and 9) is:
CH3CO2NHNH2→+N2+2H−+2e−
[0041] In such an embodiment, when switch 24 is closed...
example 2
[0042] In another embodiment, a nitrogen generator was assembled according to FIG. 2 and consisted of: [0043] (a) an external electronic circuit comprising switch 24 with a resistor 22, which may be a variable resistor; [0044] (b) an electrochemical cell 23 divided by a cation membrane 29 (such as the sulfonated perfluoroethylene polymer sold under the trademark NAFION 324 by E.I. DuPont & DeNemours Co., Wilmington, Del., U.S.A., or equivalents thereof) with: [0045] i) a catholyte 27 of a solution of sodium bromate in aqueous sulphuric acid; [0046] ii) an anolyte mixture of sodium azide (about 0.1 to 4M), sodium bicarbonate (about 0.1 to 1M), sodium iodide (about 0.1 to 1M) and sodium thiocyanate (about 0.1 to 1M) in water; [0047] iii) electrodes of Nylon™ impregnated graphite fibre and GRAFOIL (such as the product sold under the trade-mark GRAFOIL™ GTB by Union Carbide Corp.).
[0048] The putative reaction at the cathode is:
BrO?+6H?+6e−→Br−+3 H20
[0049] This cell showed open circuit ...
example 3
[0050] In an alternative embodiment, a nitrogen generator was assembled according to FIG. 2 and consisted of: [0051] (a) an external electronic circuit comprising switch 24 with a resistor 22, which may be a variable resistor; [0052] (b) a cathode 25 of graphite in contact with an oxidant 27 consisting of a paste of manganese dioxide in aqueous sulphuric acid (about 1 to 4M); [0053] (c) an anolyte mixture 31 of oxalic dihydrazide (about 0.1 to 3M) in aqueous sulphuric acid (about 0.1 to 1M); [0054] (d) an anode 21 of graphite.
[0055] On open circuit, this cell showed a voltage of 0.8 volt and no gas was generated at either electrode over a period of several days. When the circuit was closed, gas (nitrogen) was generated at the anode. The probable (but unknown) electrode reactions are:
Anode: H2NNHCOCONHNH2→CO22N?+4H−+4e−
Cathode: MnC2−4H−−2e−→Mn?−2H2O
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com