High speed parallel molecular nucleic acid sequencing
a nucleic acid and parallel technology, applied in the field of automatic method for sequencing nucleic acids, can solve the problems of phosphodiester bond not being formed with the next incoming dntps, termination of the growing complementary dna chain, and consuming enormous resources, and achieves the effect of reducing the shearing of sample nucleic acids and being readily automated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment
DETAILED EMBODIMENT
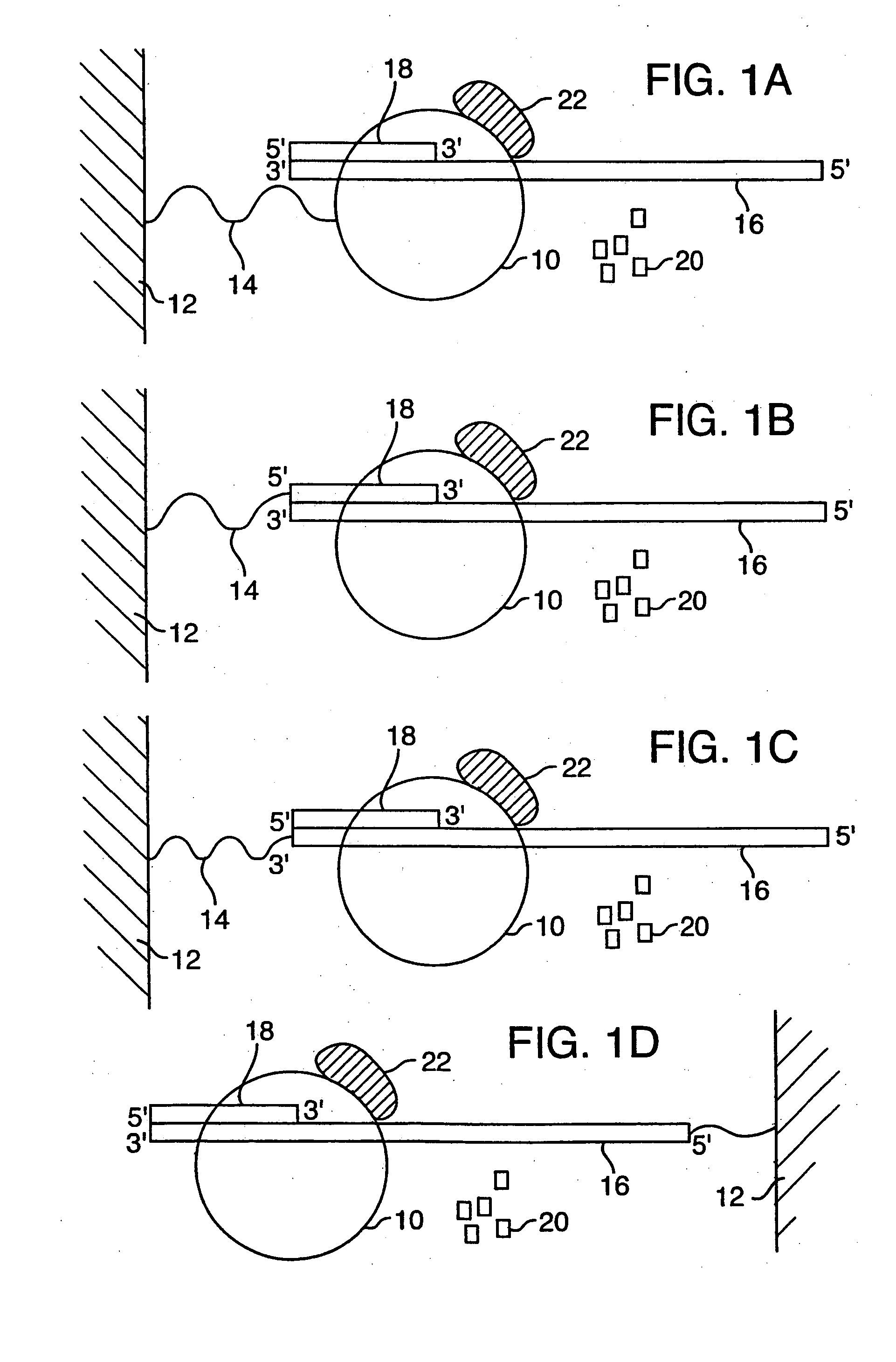
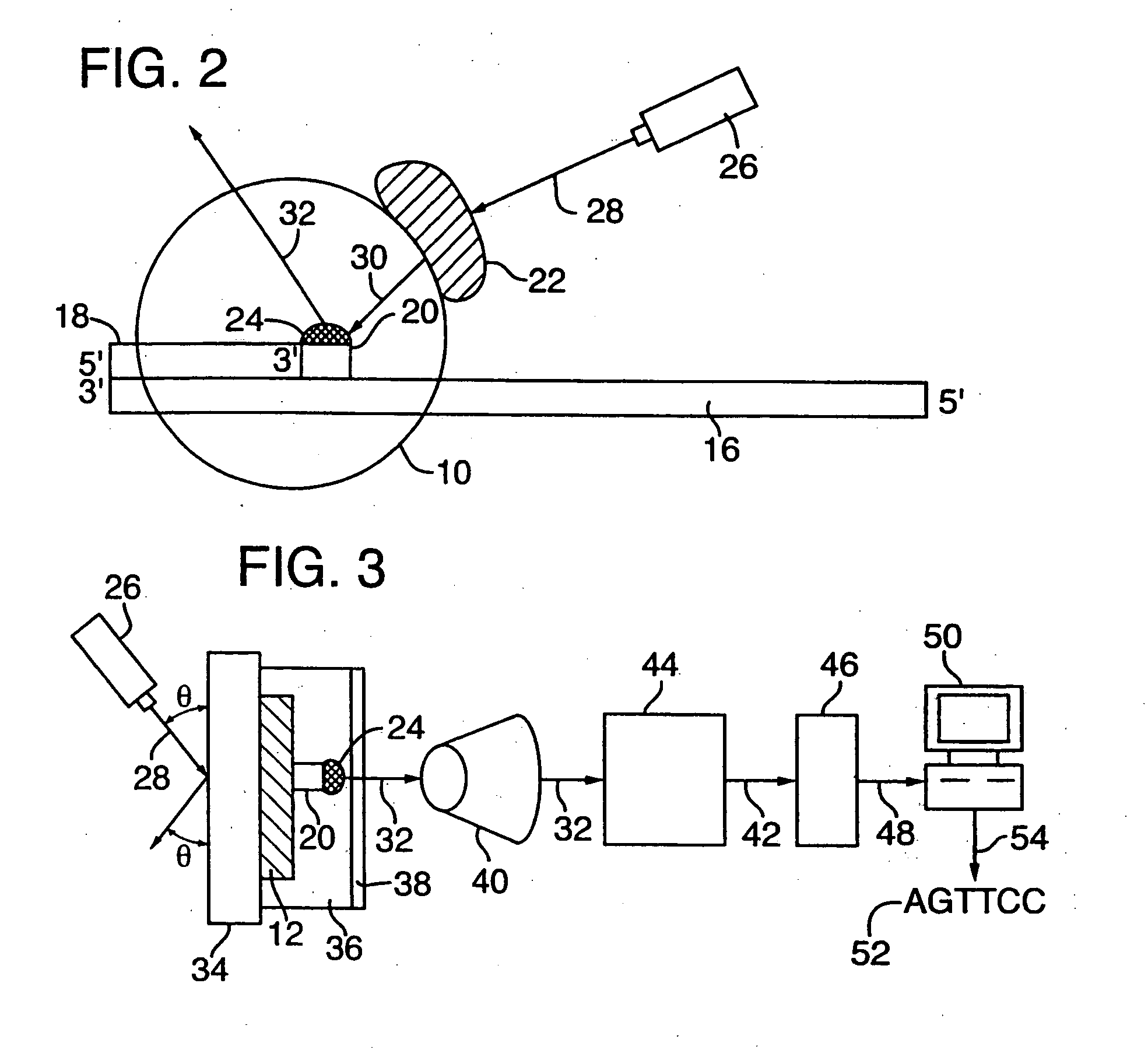
[0099] Disclosed herein is a new method for sequencing nucleic acids, and one disclosed embodiment is called Two Dye Sequencing (TDS), because it depends on at least two classes of fluorophores, a donor and an acceptor. The donor fluorophore is on a polymerase, and the acceptor fluorophore is on the nucleotides which are incorporated into the nucleic acid as a complementary strand is generated (FIGS. 1-3). In one embodiment, as shown in FIG. 1A, a polymerase 10, is attached to a substrate 12, such as a microscope slide, by a linker 14. The nucleic acid 16 to be sequenced has an annealed oligonucleotide primer 18, and is bound by the anchored polymerase 10. To start the sequencing reaction, a mixture of nucleotides 20 is added. The polymerase 10 then sequentially adds the appropriate nucleotide 20 to the complementary strand. As shown in FIG. 3, the substrate 12, can be mounted onto a microscope stage 34. The sequencing reaction may take place in an aqueous environ...
example 1
Preparation of Fluorescent or Luminescent Polymerases
[0109] This example describes how to prepare polymerases containing at least one fluorophore or luminescent molecule. The fluorophore or luminescent molecule may be a donor fluorophore.
Recombinant GFP-polymerase
[0110] Green fluorescent protein (GFP) includes a chromophore formed by amino acids in the center of the GFP. GFP is photostable, making it a desirable fluorophore to use on the polymerase, because it is resistant to photobleaching during excitation. Wild-type GFP is excited at 393 nm or 476 nm to produce an emission at 508 nm.
[0111] GFP mutants have alternative excitation and emission spectra. One GFP mutant, H9-40 (Tsien, 1998, Ann. Rev. Biochem. 67:509; U.S. Pat. Nos. 5,625,048 and 5,777,079 to Tsien and Heim, herein incorporated by reference), has only a single absorption at 398 nm and emits at 511 nm. A red-shifted GFP mutant RSGFP4 (Delagrave et al., Biotechnology 13:151-4, 1995) has an excitation at 490 nm and e...
example 2
Attachment of the Polymerase or Nucleic Acid to a Substrate
[0131] This example describes methods that can be used to attach the fluorescent polymerase generated in Example 1, or a nucleic acid, to a substrate, such as a microscope slide or gel matrix. During the sequencing reaction, the sample nucleic acid to be sequenced, the oligonucleotide primer, or the polymerase, is attached to a substrate in the microscope field of view.
Attachment of Nucleic Acids
[0132] Several methods for attaching nucleic acids (for example the sample nucleic acid to be sequenced or an oligonucleotide primer) to a substrate are available. In particular embodiments, nucleic acids can be attached by their 5′ or 3′ end, or anywhere in between. For example, a 5′ biotinylated primer can be synthesized (Beaucage, Tetrahedron Letters 22:1859-62, 1981; Caruthers, Meth. Enzym. 154:287-313, 1987), and affixed to a streptavidin coated substrate surface (Hultman, Nucl. Acids Res. 17:493746, 1989). In another embodi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance wavelength | aaaaa | aaaaa |
| absorbance wavelength | aaaaa | aaaaa |
| absorbance wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com