Method for treatment of HIV infection
a hiv infection and treatment method technology, applied in the field of new medicines, can solve the problems of difficult continuous use of anti-hiv drugs, inability to observe inflammatory responses, and inability to take anti-hiv drugs, so as to reduce side effects, prevent infection, and maintain low to undetectable viral loads
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
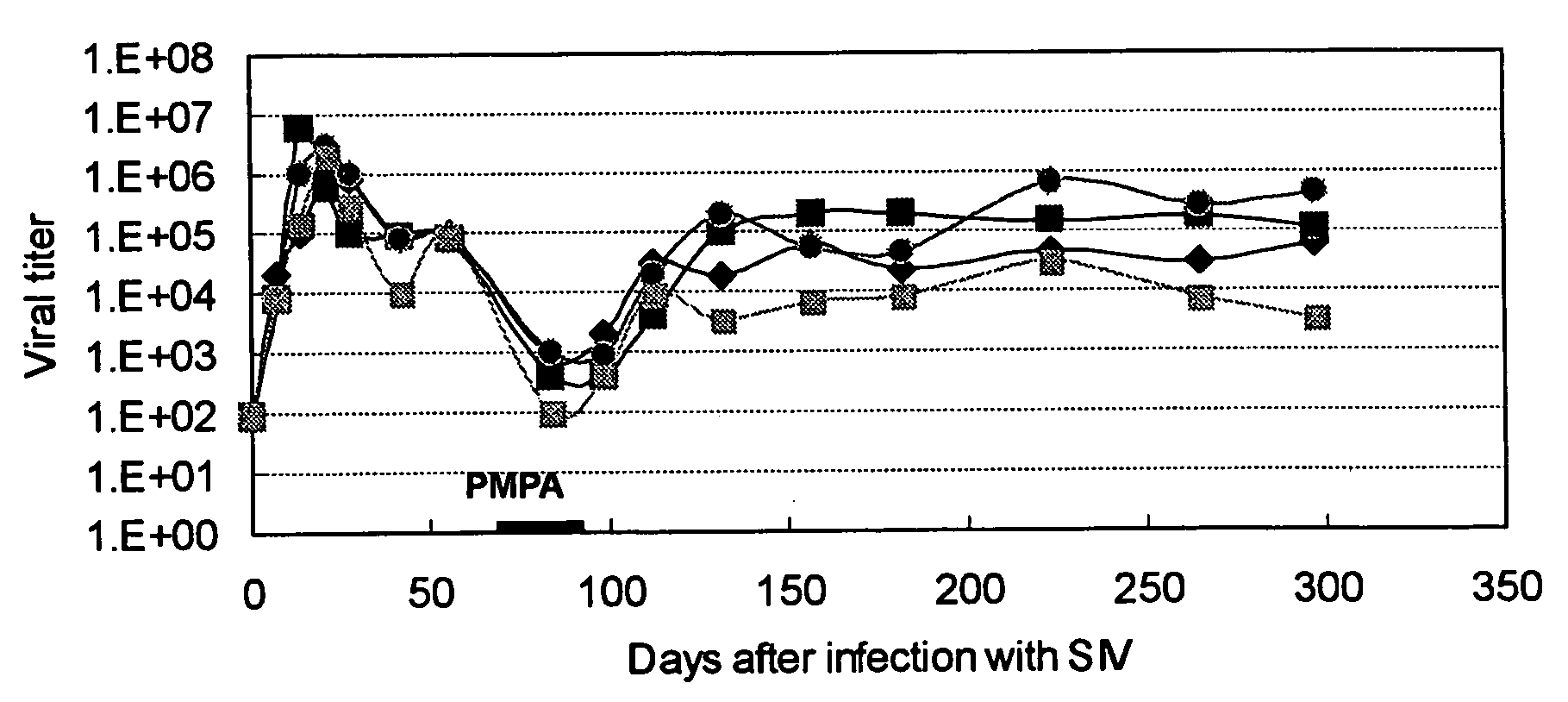
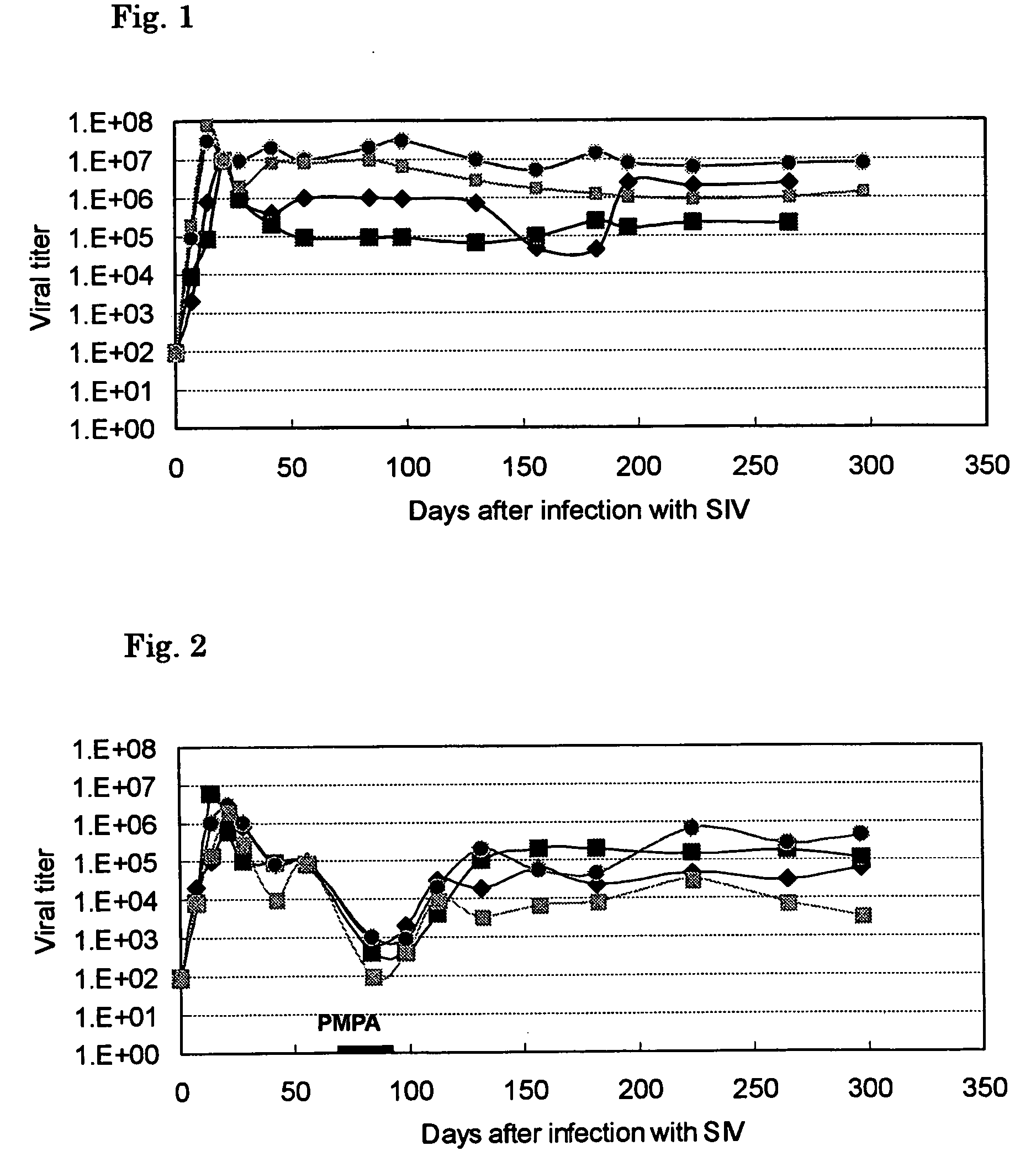
Image
Examples
example 1
[0032] Skins of healthy adult rabbits were inoculated with vaccinia virus to cause inflammation. The inflammatory skins were removed, finely cut and phenol water was added thereto. The mixture was filtered with pressure, and the resulting filtrate was adjusted to pH 5 with hydrochloric acid and then heated at 90-100° C. for 30 minutes. Proteins were removed by filtration, the filtrate was adjusted to pH 9 with sodium hydroxide, further heated at 90-100° C. for 15 minutes and filtered. The filtrate was adjusted to about pH 4, stirred for 2 hours after adding 2% of activated charcoal, and centrifuged. The resulting activated charcoal was mixed with water, adjusted to pH 10 with sodium hydroxide, stirred at 60° C. for 1.5 hours and centrifuged to give a supernatant. The activated charcoals precipitated by centrifugation were mixed with water, adjusted to pH 11 with sodium hydroxide, stirred at 60° C. for 1.5 hours and centrifuged to give a supernatant. Both of the supernatants obtained...
example 2
[0033] Skins of healthy adult rabbits were inoculated with vaccinia virus to cause inflammation. The inflammatory skins were aseptically removed, finely cut and phenol-added glycerin water was added thereto. The mixture was ground using a homogenizer to prepare an emulsion. The emulsion was filtered with centrifugation, and the resulting filtrate was adjusted to pH 4.8-5.5 with hydrochloric acid, heated at 100° C. with a steam flow and then filtered. The filtrate was further filtered with Seitz filter, adjusted to pH 9.2 with sodium hydroxide, heated at 100° C. and filtered. The filtrate was adjusted to pH 4.5, stirred for 1-5 hours after adding 1.5% of activated charcoal, and filtered. The activated charcoal was mixed with water, adjusted to pH 9.4-10 with sodium hydroxide, stirred for 3-5 hours and filtered. The resulting filtrate was neutralized with hydrochloric acid and dried in vacuo.
example 3
[0034] Skins of healthy adult rabbits were inoculated with vaccinia virus to activate or stress the tissues. The activated skins were aseptically removed, finely cut and water was added thereto. The mixture was ground using a homogenizer to prepare an emulsion. The emulsion was filtered with pressure, and the resulting filtrate was adjusted to pH 5.0 with hydrochloric acid and heated at 100° C. with a steam flow. Proteins were removed by filtration, the filtrate was adjusted to pH 9.1 with sodium hydroxide, heated at 100° C. and filtered. The filtrate was adjusted to pH 4.1, stirred after adding 2% of activated charcoal, and the mixture was filtered to obtain a filtrate and a first batch of recovered activated charcoal. To the filtrate was added 5.5% of activated charcoal and the mixture was stirred for 2 hours, and filtered to obtain a second batch of recovered activated charcoal. The first batch of recovered activated charcoal was mixed with water, adjusted to pH 9.9 with sodium h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com