Methods for sterilizing preparations of digestive enzymes
a technology of digestive enzymes and preparations, which is applied in the direction of macromolecular non-active ingredients, lavatory sanitory, pharmaceutical non-active ingredients, etc., can solve the problems of poor sorption, insufficient nutrient-rich substances, and insufficient penetration of ingested meats, so as to reduce the temperature of the preparation, and reduce the residual solvent content of the preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
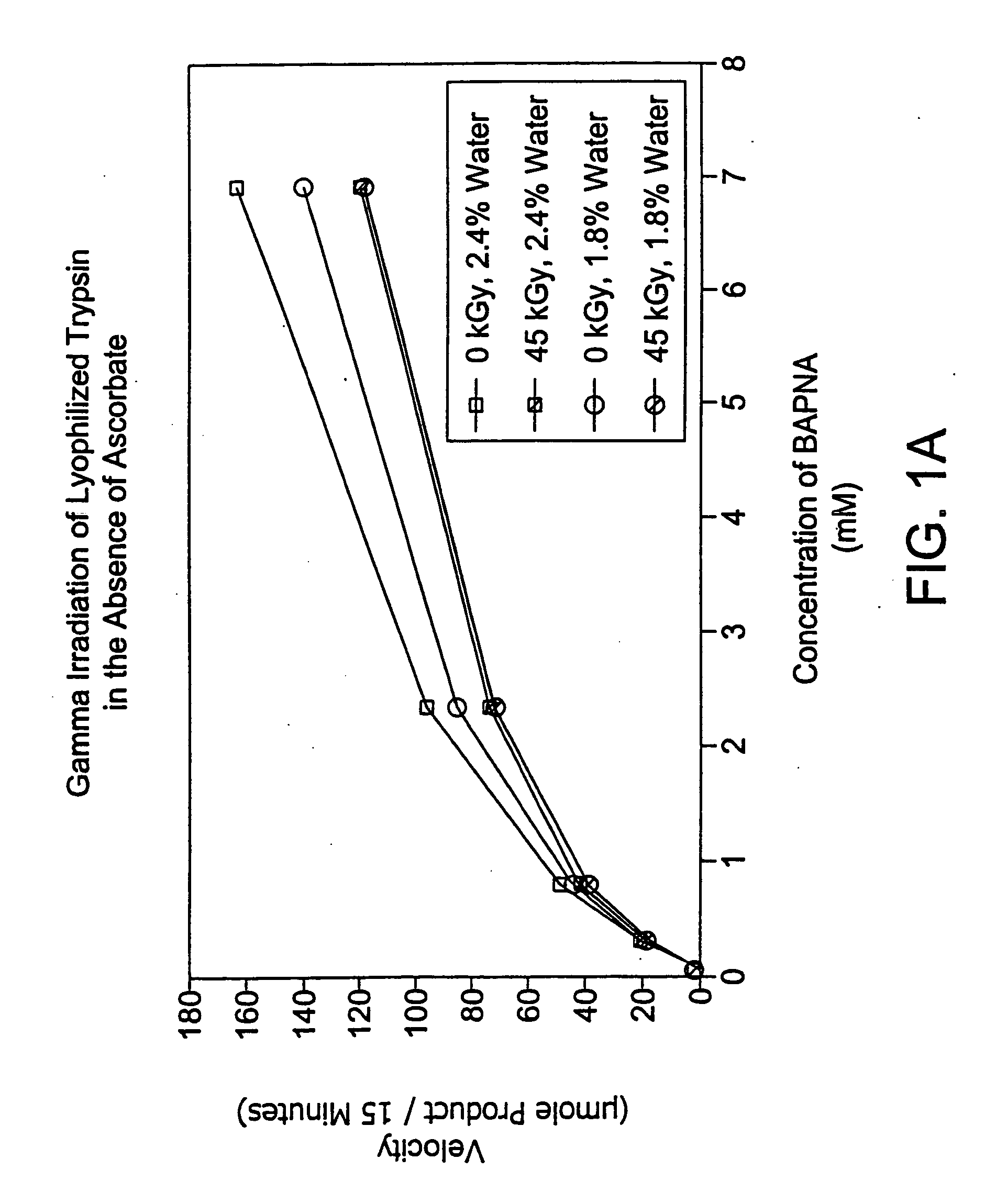
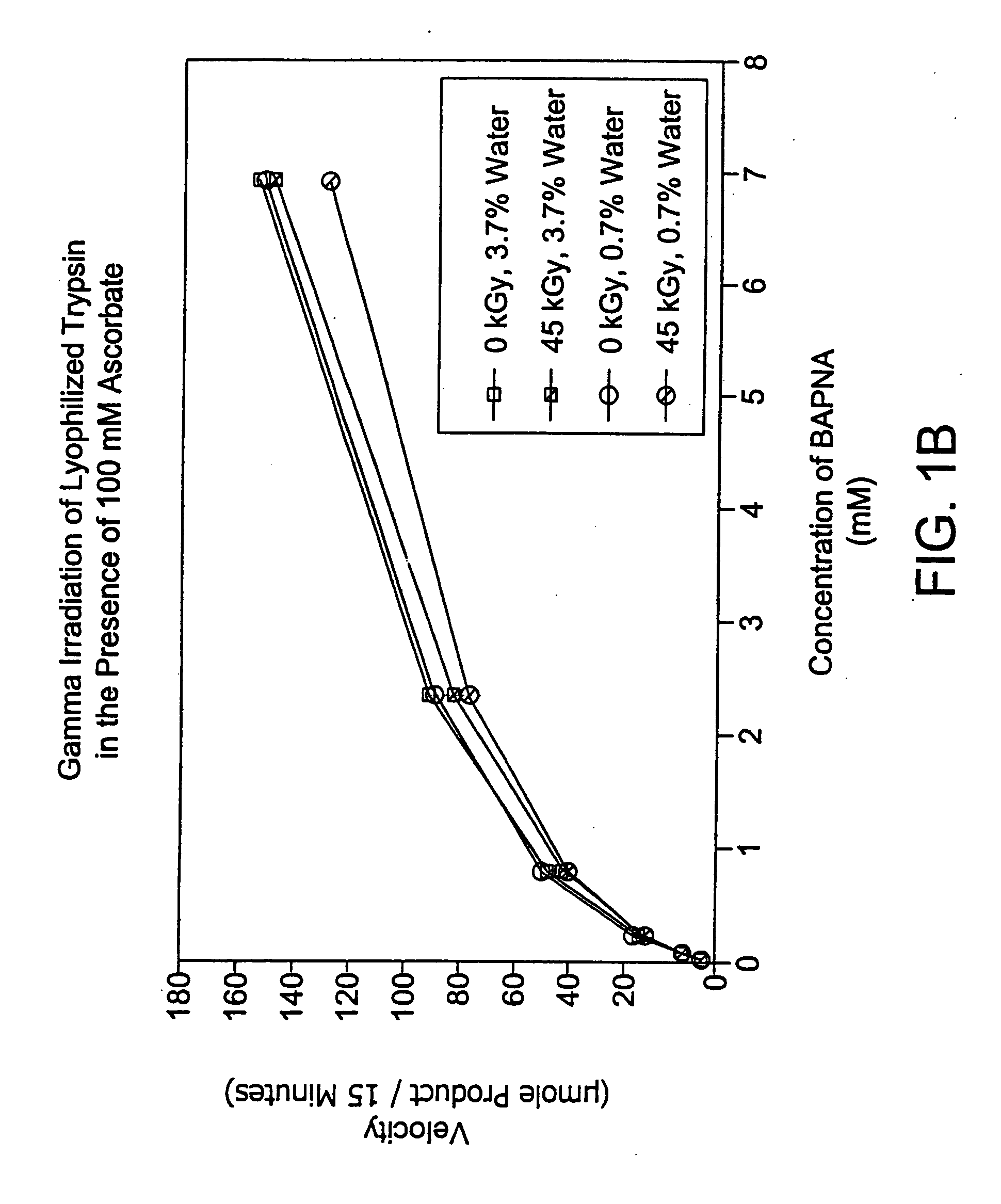
[0107] In this experiment, lyophilized trypsin was irradiated (45 kGy at 1.9 kGy / hr) alone or in the presence of a stabilizer (sodium ascorbate 100 mM) at varying levels of residual solvent content.
Method
[0108] 1 ml aliquots of trypsin alone or with 100 mM sodium ascorbate (10 mg / ml) were placed in 3 ml vials. Samples were prepared in triplicate and subjected to lyophilization, either a primary drying cycle (22 hours, sample temp 0-10° C., shelf temp 35° C., 10 mT) or a combination of a primary drying cycle and a secondary drying cycle (60 hours, sample temp 40° C., shelf temp 40° C., 10 mT).
[0109] All samples were resuspended in 1 ml water, and then diluted 1:10 for assay. Assay conditions: 50 units / ml trypsin per well+BAPNA substrate starting at 3000 μg / ml was serially diluted 3-fold down a 96-well plate. The assay was set up in two 96-well plates and absorption read at both 405 and 620 nm at 5 and 20 minutes. The absorption at 630 nm (background) was subtracted from the value...
example 2
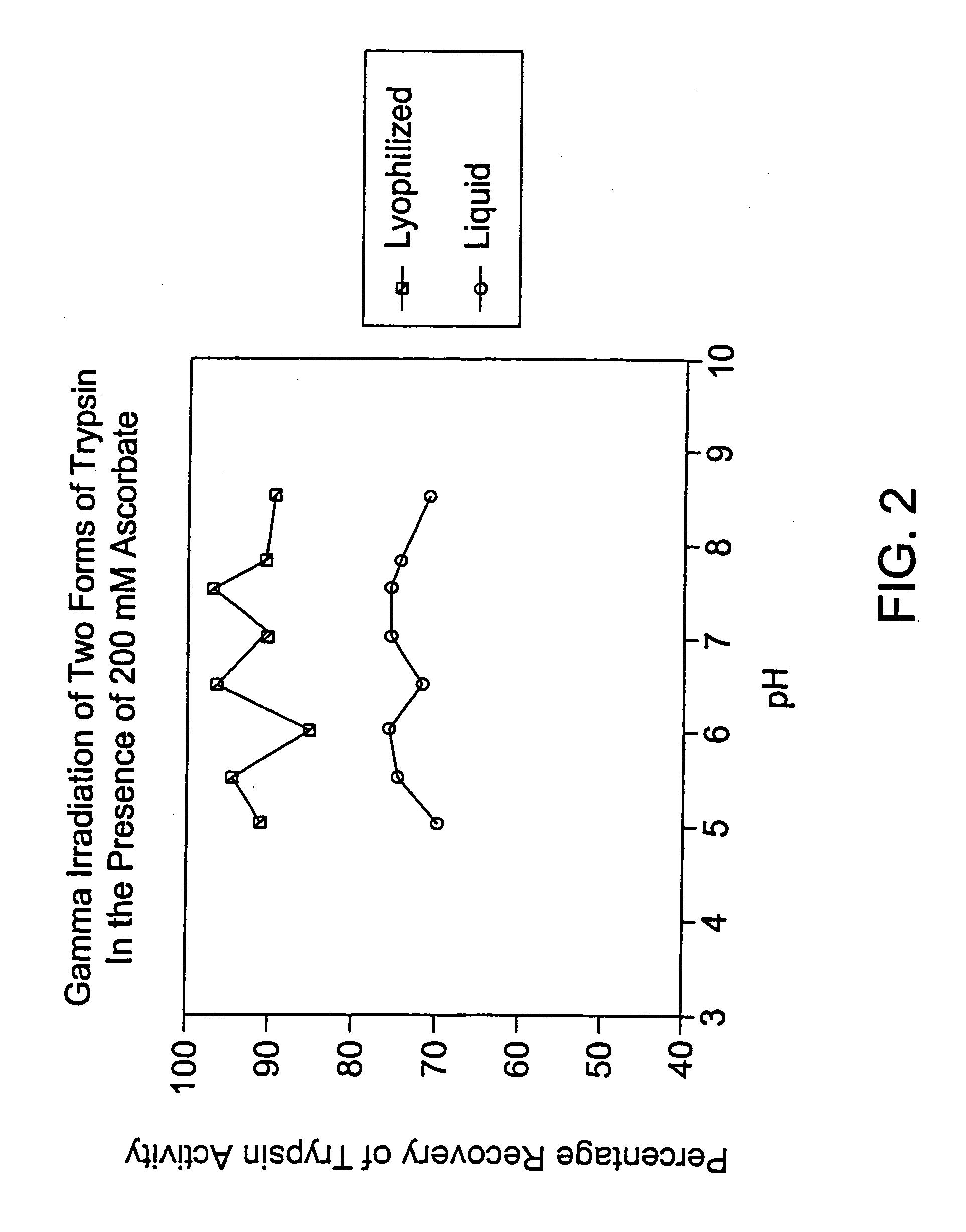
[0113] In this experiment, trypsin was irradiated (45 kGy at 1.6 kGy / hr. and 4° C.) in the presence of a stabilizer (sodium ascorbate 200 mM) as either a liquid or lyophilized preparation at varying pH levels.
Method
[0114] 1 ml of 1 mg / ml (about 3000 IU / ml) trypsin aliquots in the presence of 35 mM phosphate buffer and 200 mM sodium ascorbate were made at varying pH levels between 5 and 8.5, inclusive. 400 μl of each solution was placed in 3 ml vials and then lyophilized and gamma-irradiated. The remaining portion of each solution was gamma-irradiated as a liquid. Lyophilized and liquid samples were assayed at the same time, under the following conditions: Assay conditions: 5 U / well trypsin (50 U / ml)+BATNA substrate (1 mg / ml) was serially diluted 3-fold down a 96-well plate. The assay was set up in two 96-well plates and absorption read at both 405 and 620 nm at 5 and 20 minutes. The absorption at 630 nm (background) was subtracted from the value at 405 nm to obtain a corrected ab...
example 3
[0116] In this experiment, lyophilized trypsin was irradiated (42.7-44.8 kGy at 2.65 kGy / hr at 4° C.) alone or in the presence of a stabilizer (sodium ascorbate 200 mM).
Method
[0117] 1 ml aliquots of trypsin alone or with 200 mM sodium ascorbate (1 mg / ml) were placed in 3 ml vials and frozen overnight at −70° C. Samples were prepared in quadruplicate and subjected to lyophilization, utilizing primary and secondary drying cycles (20 hours total).
[0118] All samples were resuspended in 1 ml water, and then diluted 1:10 for assay. Assay conditions: 50 units / ml trypsin per well+BATNA substrate starting at 3000 μg / ml was serially diluted 3-fold down a 96-well plate. The assay was set up in two 96-well plates and absorption read at both 405 and 620 nm at 5 and 20 minutes. The absorption at 630 nm (background) was subtracted from the value at 405 nm to obtain a corrected absorption value. The change in this value over time between 5 and 15 minutes of reaction time was plotted and Vmax an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com