Specific antibody directed to active hepatocyte growth factor activator and method for using the same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Inactive HGFA and Active HGFA
[0108] Inactive HGFA was prepared by using HGFA cDNA coding for the HGFA precursor described in Japanese Patent Laid-open Publication No. 6-153946 according to the method described in Japanese Patent Laid-open Publication No. 6-153966 to produce recombinant inactive HGFA. That is, the HGFA cDNA coding for the full length of the inactive HGFA precursor, 655 amino acids, was inserted into pcDNA3.1 (Invitrogen), which is an animal cell expression vector, downstream from a CMV promoter in a conventional manner.
[0109] The obtained expression vector was introduced into a CHO cell, which is an animal cell strain derived from a Chinese hamster ovary cell, by using a Transfectam (BioSepra) according to the attached instruction. Then, CHO cells expressing HGFA cDNA were selected by utilizing a property of a neomycin resistant gene existing on the introduced expression vector. That is, cells that could grow in an ERDF medium (Kyokuto Seiyaku) conta...
example 3
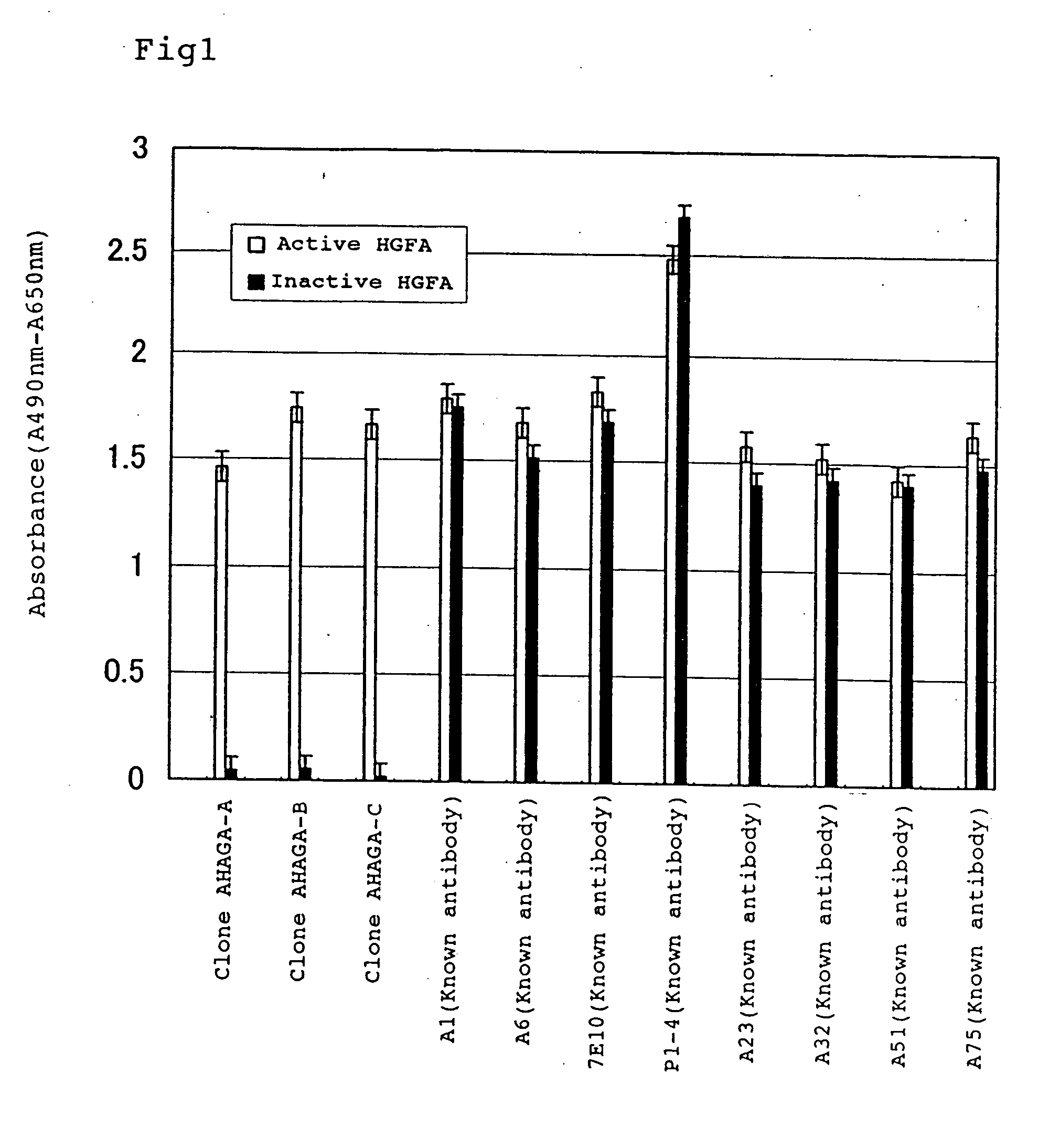
Preparation of Active HGFA Specific Monoclonal Antibody
[0115] A solution containing 100 μg of the 36 kDa HGFA or 100 μg of the 98 kDa HGFA, which were prepared in Example 1, was subcutaneously and intraperitoneally administered to a Balb / c mouse with the same volume of complete Freund's adjuvant 6 times with 2-week intervals. After production of antibodies in the serum of the mouse was confirmed, a solution containing 100 μg of HGFA was administered into the caudal vein. Three days later, spleen was removed and spleen cells were fused with myeloma cells P3U1 by using polyethylene glycol 1500 according to “Monoclonal Antibody Experimental Manual” (Kodansha Scientific, 1987), introduced into wells of 96-well plate, added with HAT medium and cultured for 14 days.
[0116] Subsequently, hybridomas producing monoclonal antibodies specific to respective active HGFA in the medium were selected. That is, culture supernatant of a hybridoma subjected to selection was added to an ELISA plate fo...
example 5
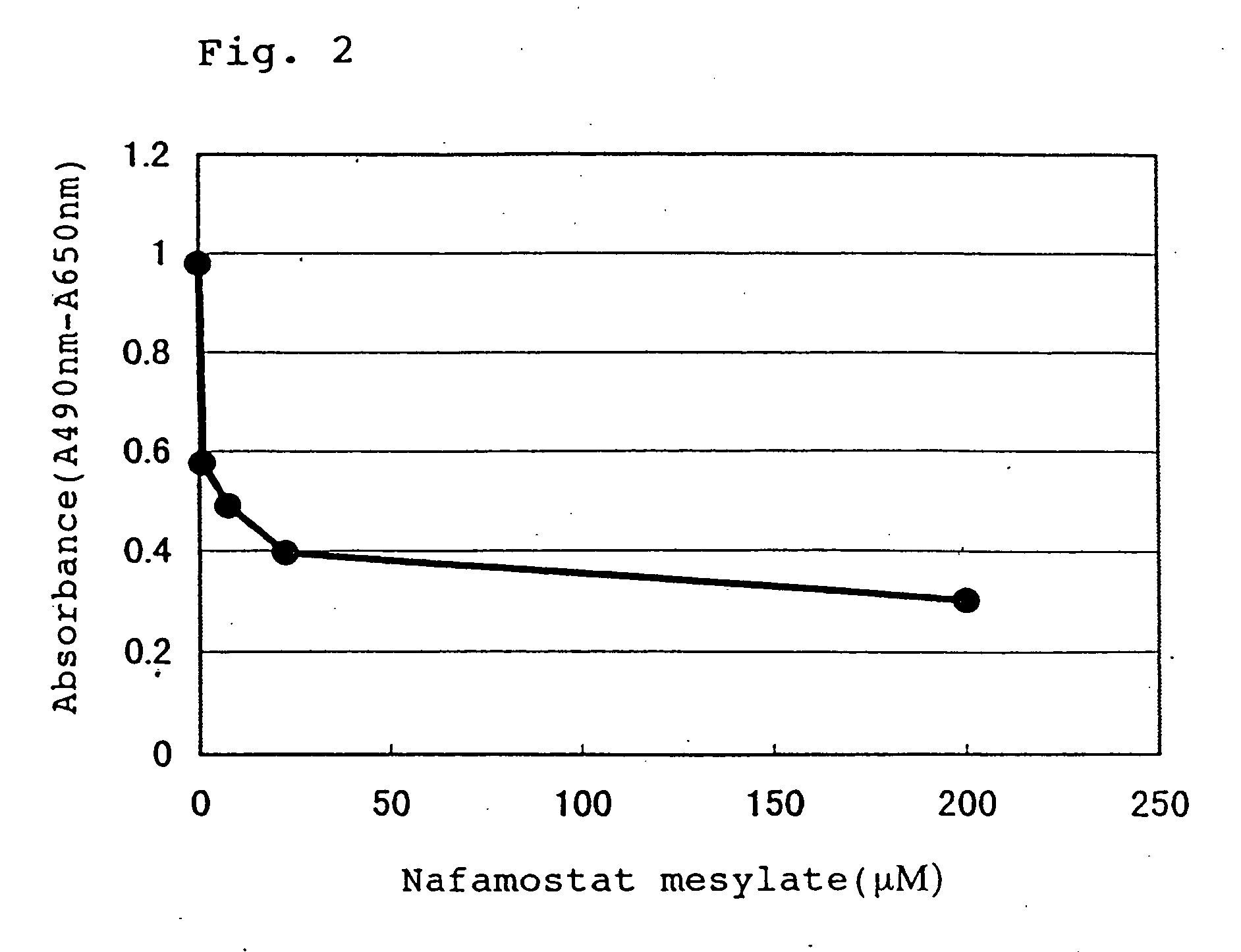
Measurement of Dissociation Constant of Active HGFA Specific Monoclonal Antibody
[0123] Among active HGFA specific monoclonal antibodies prepared and purified in the Example 3, the dissociation constant was measured for the monoclonal antibody derived from the hybridoma clone AHGA-A. An active HGFA immobilized plate on which active HGFA was immobilized and blocked with BSA was added with the antibodies at different antibody concentrations and allowed to sufficiently react until equilibrium was reached (2 hours or longer).
[0124] Subsequently, the plate was sufficiently washed with a PBS(-) solution containing 0.05% Tween 20 (hereafter, abbreviated as “PBST solution”), and then 100 μl of PBS(-) containing 1 μg / ml of HRP (horseradish peroxidase) conjugated goat anti-mouse IgG / Fc polyclonal antibody (ICN) and 1% BSA was added to each well and further allowed to react at room temperature for 1 hour. The plate was sufficiently washed with a PBST solution, and then a citrate-phosphate buf...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Biological properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com