Multipotent adult stem cells, sources thereof, methods of obtaining and maintaining same, methods of differentiation thereof, methods of use thereof and cells derived thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Selection. Culture and Characterization of Mouse Multipotent Adult Stem Cells (mMASC)
Cell Isolation and Expansion
[0093] All tissues were obtained according to guidelines from the University of Minnesota IACUC. BM mononuclear cells (BMMNC) were obtained by ficoll-hypaque separation of BM was obtained from 5-6 week old ROSA26 mice or C57 / BL6 mice. Alternatively, muscle and brain tissue was obtained from 3-day old 129 mice. Muscles from the proximal parts of fore and hind limbs were excised from and thoroughly minced. The tissue was treated with 0.2% collagenase (Sigma Chemical Co, St Louis, Mo.) for 1 hour at 37° C., followed by 0.1% trypsin (Invitrogen, Grand Island, N.Y.) for 45 minutes. Cells were then triturated vigorously and passed through a 70-urn filter. Cell suspensions were collected and centrifuged for 10 minutes at 1600 rpm. Brain tissues was dissected and minced thoroughly. Cells were dissociated by incubation with 0.1% trypsin and 0.1% DNAse (Sigma) for 30 minutes at ...
example 2
Selection and Culture of Rat Multipotent Adult Stem Cells (rMASC)
[0103] BM and MNC from Sprague Dawley or Wistar rats were obtained and plated under conditions similar for mMASC. After 21-28 days, cells were depleted of CD45+ cells, and the resulting CD45 cells were subcultured at 10 cells / well.
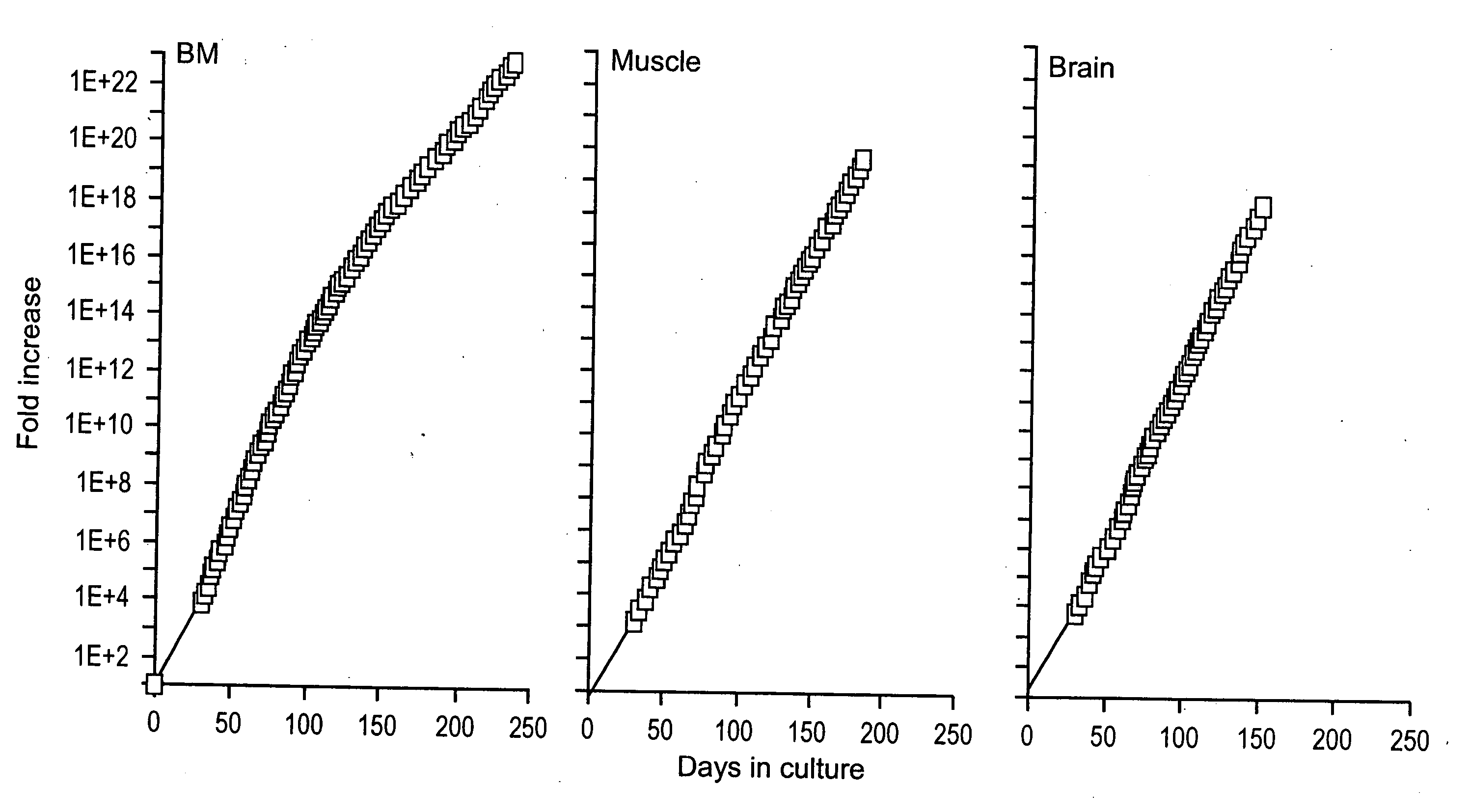
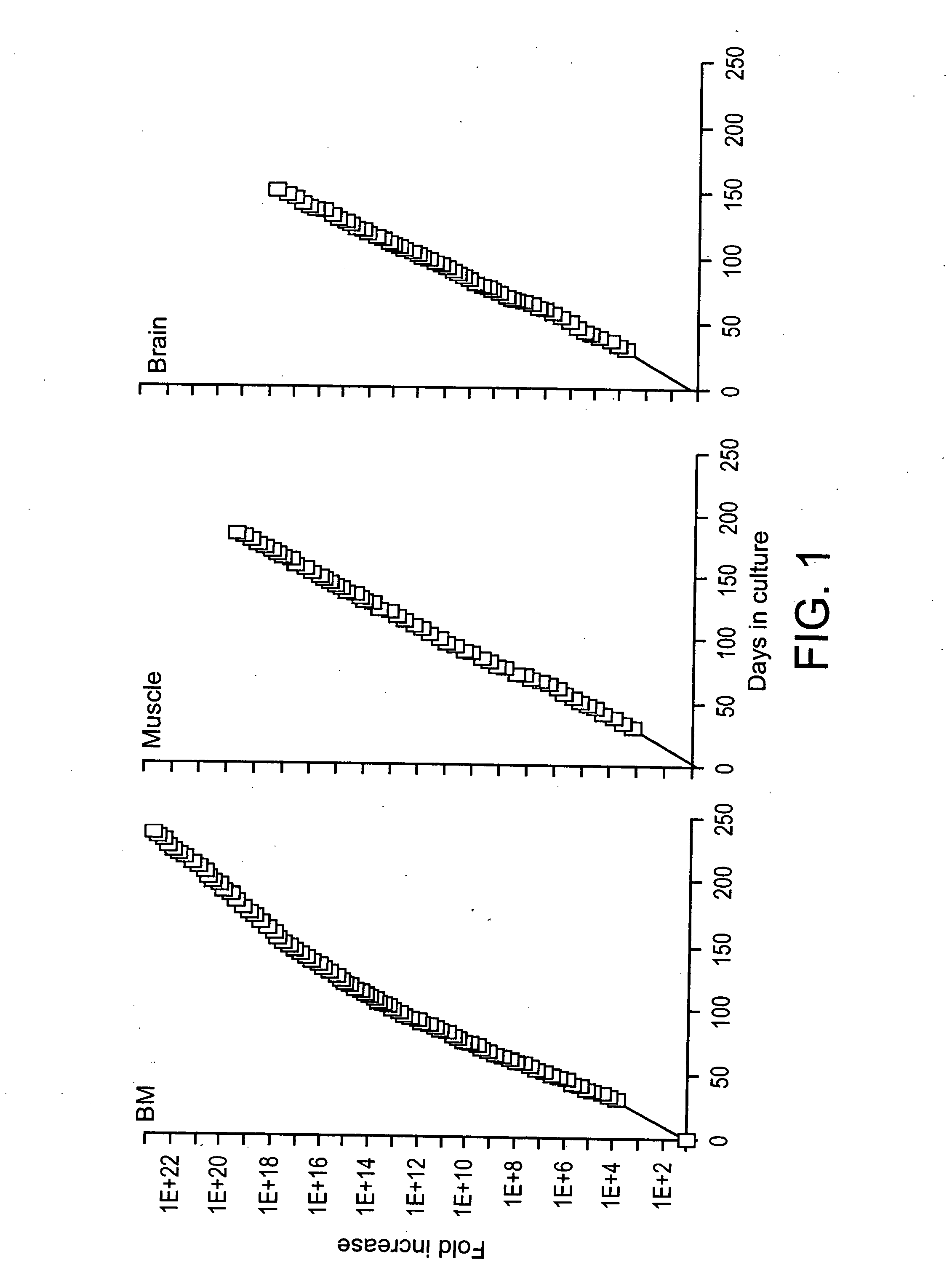
[0104] Similar to mMASC, rMASC have been culture expanded for >100 PDs. Expansion conditions of rat MASC culture required the addition of EGF, PDGF-BB and LIF and culture on FN, but not collagen type 1, laminin or Matrigel™. rMASC were CD44, CD45 and MHC class I and II negative, and expressed high levels of telomerase. The ability of a normal cell to grow over 100 cell doublings is unprecedented, unexpected and goes against conventional dogma of more than two decades.
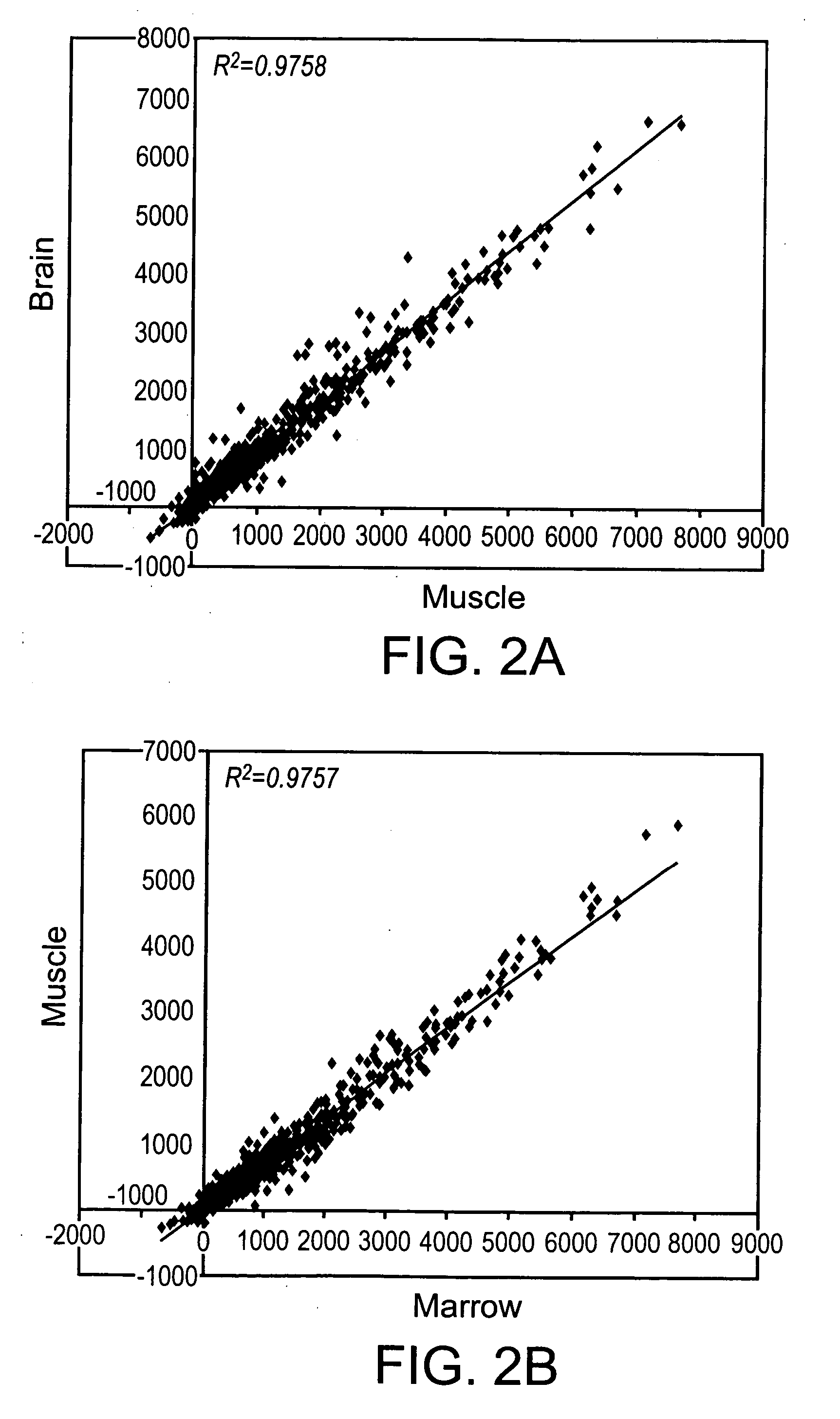
[0105] Rat MASC that had undergone 42 PDs, 72 PDs, 80 PDs, and 100 PDs, were harvested and telomere lengths evaluated. Telomeres did not shorten in culture, as was determined by Southern blot analysis after 42 PDs, 72 PDs, 80 ...
example 3
Selection and Culture of Human Multipotent Adult Stem Cells (hMASC)
[0106] BM was obtained from healthy volunteer donors (age 2-50 years) after informed consent using guidelines from the University of Minnesota Committee on the use of Human Subject in Research. BMMNC were obtained by Ficoll-Paque density gradient centrifugation and depleted of CD45+ and glycophorin-A+ cells using micromagnetic beads (Miltenyii Biotec, Sunnyvale, Calif.).
[0107] Expansion conditions: 5×103 CD45− / GlyA− cells were diluted in 200 μL expansion medium [58% DMEM-LG, 40% MCDB-201 (Sigma Chemical Co, St Louis, Mo.), supplemented with 1× insulin-transferrin-selenium (ITS), 1×-linoleic-acid bovine serum albumin (LA-BSA), 10−8 M Dexamethasone, 10−4 M ascorbic acid 2-phosphate (all from Sigma), 100 U penicillin and 1,000 U streptomycin (Gibco)] and 0-10% fetal calf serum (FCS) (Hyclone Laboratories, Logan, Utah) with 10 ng / ml of EGF (Sigma) and 10 ng / ml PDGF-BB (R&D Systems, Minneapolis, Minn.)] and plated in we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com