Human CTLA-4 antibodies and their uses
a human ctla4 and human sequence technology, applied in the field of molecular immunology and the treatment of human diseases, can solve problems such as acute toxicity, and achieve the effects of enhancing or suppressing or prolonging an immune response, reducing the risk of infection, and improving the immune respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
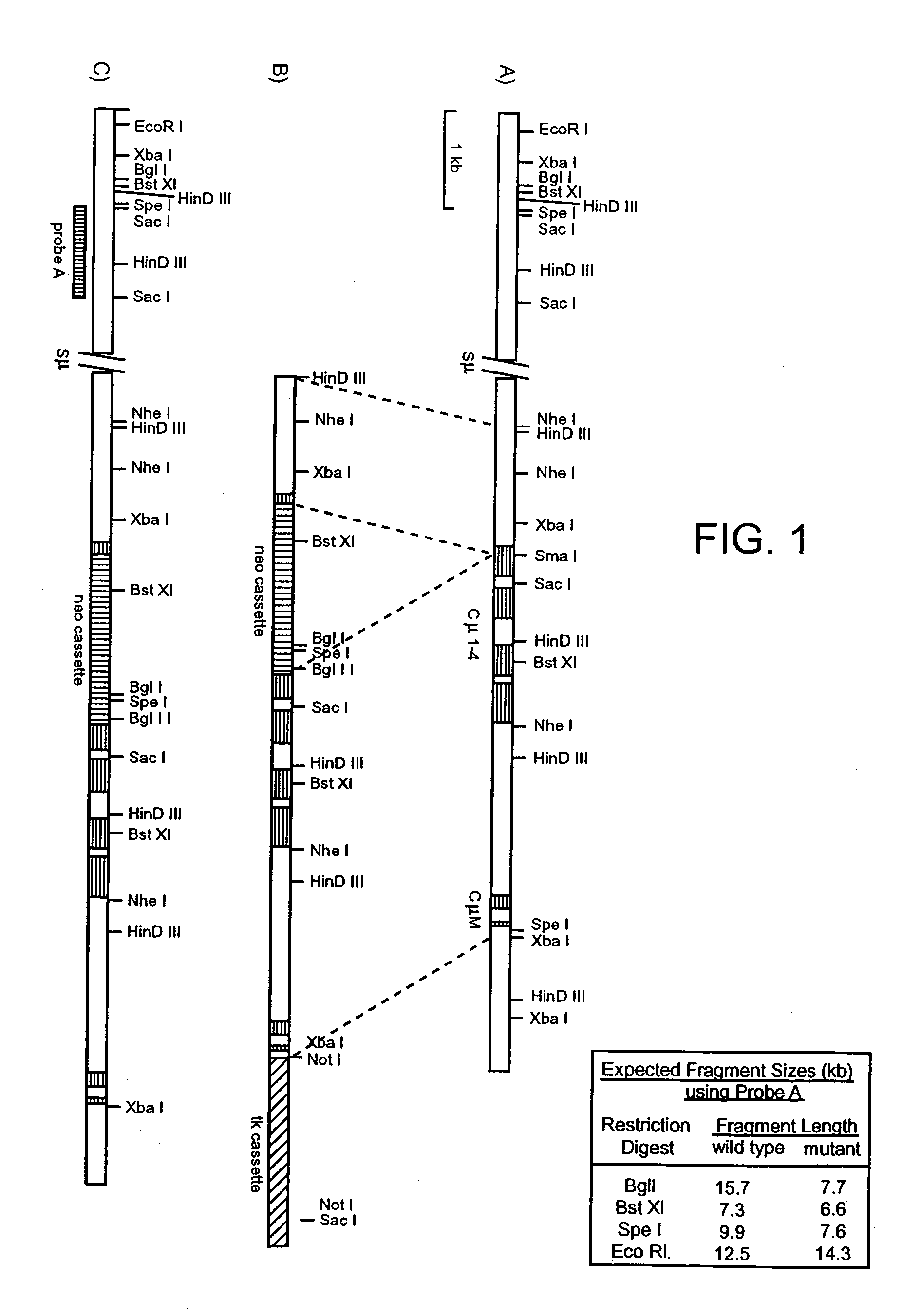
Generation of Cmu Targeted Mice
[0274] Construction of a CMD Targeting Vector.
[0275] The plasmid pICEmu contains an EcoRI / XhoI fragment of the murine Ig heavy chain locus, spanning the mu gene, that was obtained from a Balb / C genomic lambda phage library (Marcu et al. Cell 22: 187, 1980). This genomic fragment was subcloned into the XhoI / EcoRI sites of the plasmid pICEMI9H (Marsh et al; Gene 32, 481-485, 1984). The heavy chain sequences included in pICEmu extend downstream of the EcoRI site located just 3′ of the mu intronic enhancer, to the XhoI site located approximately 1 kb downstream of the last transmembrane exon of the mu gene; however, much of the mu switch repeat region has been deleted by passage in E. coli.
[0276] The targeting vector was constructed as follows (see FIG. 1). A 1.3 kb HindIII / SmaI fragment was excised from pICEmu and subcloned into HindIII / SmaI digested pBluescript (Stratagene, La Jolla, Calif.). This pICEmu fragment extends from the HindIII site located ...
example 2
Generation of HCol2 Transgenic Mice
[0284] The HCol2 Human Heavy Chain Transgene.
[0285] The HCo12 transgene was generated by coinjection of the 80 kb insert of pHC2 (Taylor et al., 1994, Int. Immunol., 6: 579-591) and the 25 kb insert of pVx6. The plasmid pVx6 was constructed as described below.
[0286] An 8.5 kb HindIII / SalI DNA fragment, comprising the germline human VH1-18 (DP-14) gene together with approximately 2.5 kb of 5′ flanking, and 5 kb of 3′ flanking genomic sequence was subcloned into the plasmid vector pSP72 (Promega, Madison, Wis.) to generate the plasmid p343.7.16. A 7 kb BamHI / HindIII DNA fragment, comprising the germline human VH5-51 (DP-73) gene together with approximately 5 kb of 5′ flanking and 1 kb of 3′ flanking genomic sequence, was cloned into the pBR322 based plasmid cloning vector pGP1f (Taylor et al. 1992, Nucleic Acids Res. 20: 6287-6295), to generate the plasmid p251f. A new cloning vector derived from pGP1f, pGP1k (Seq. ID #1), was digested with EcoRV / ...
example 3
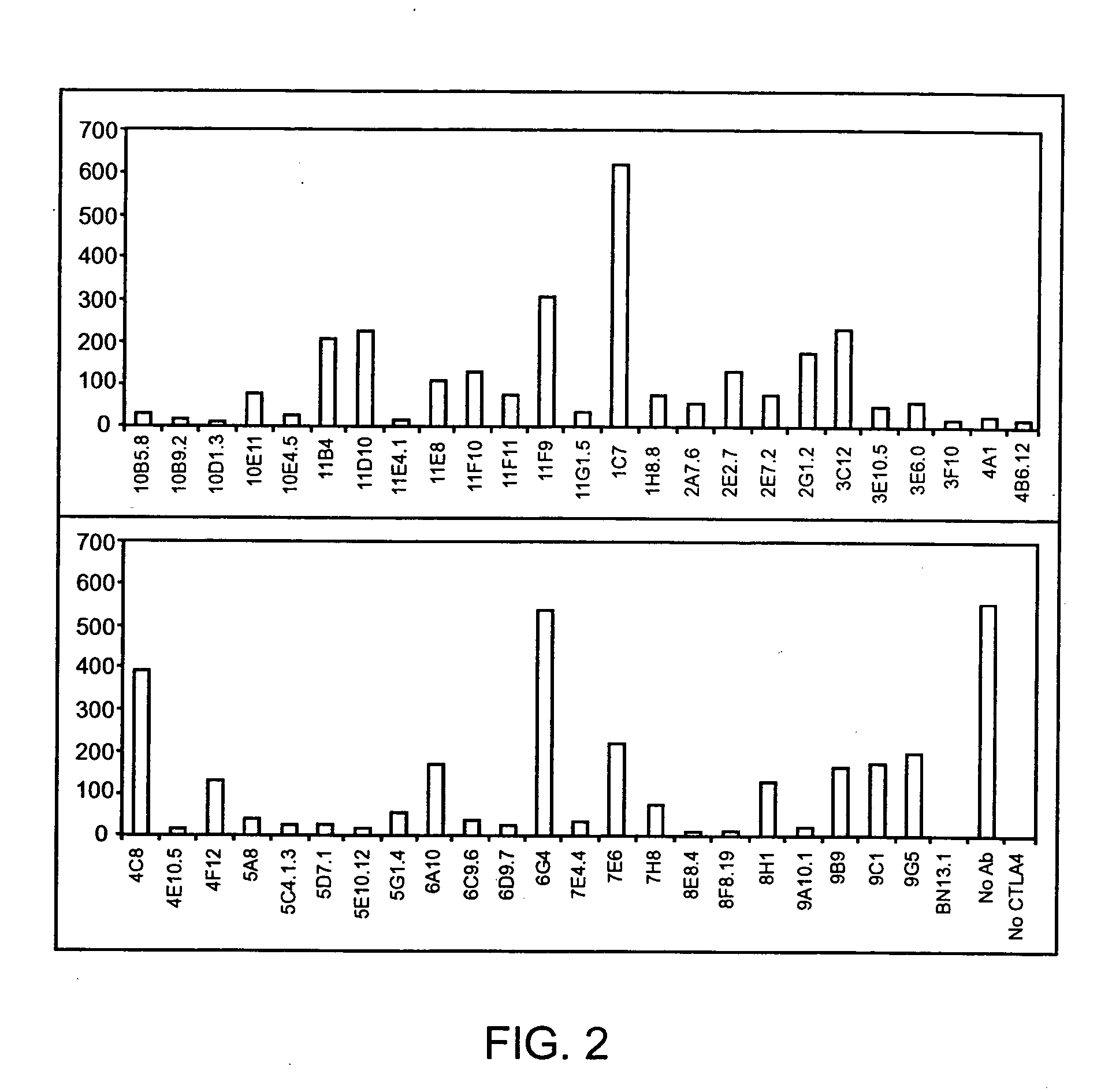
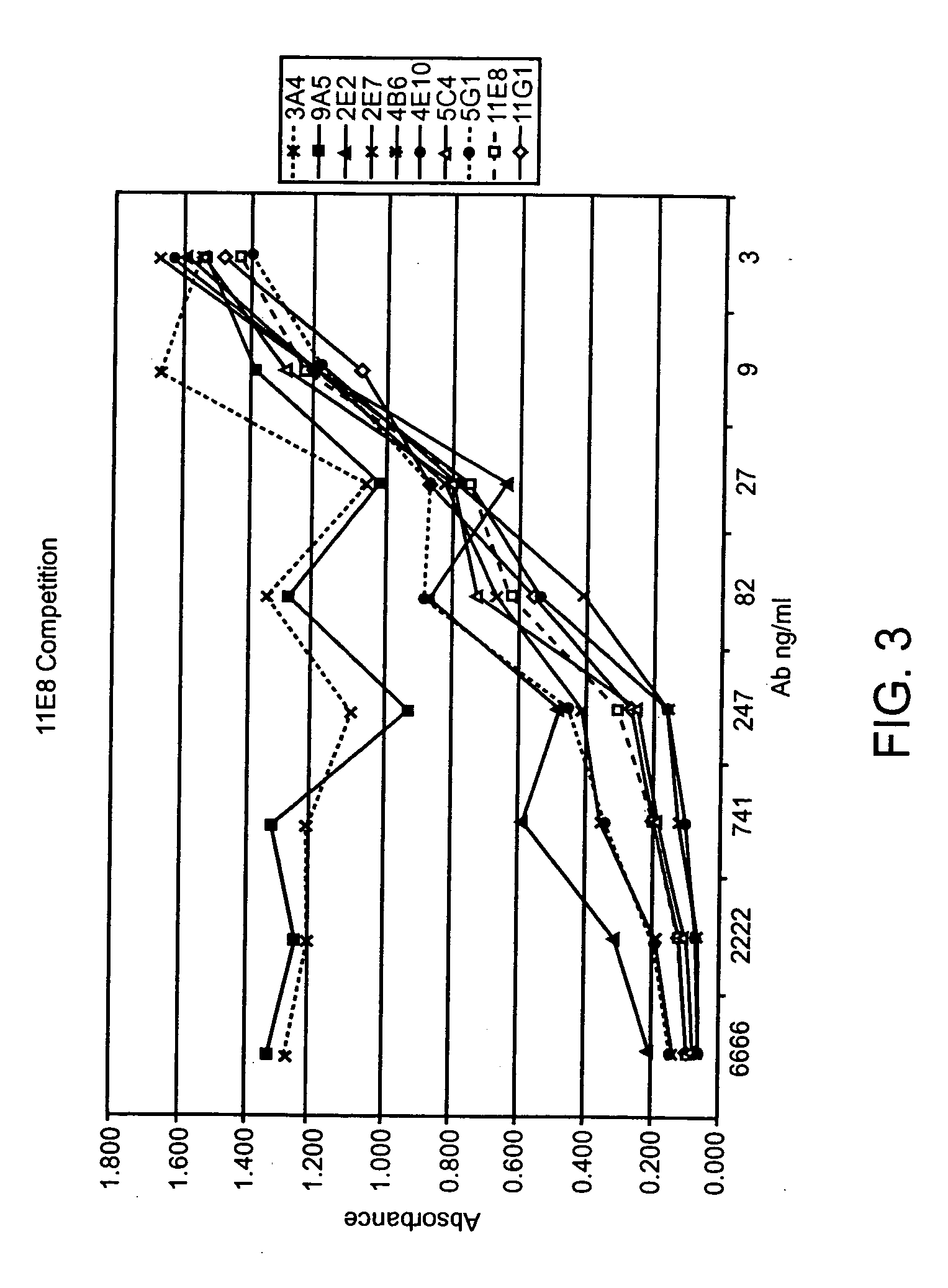
Generation of Human IgG Kappa Anti-Human CTLA-4 Monoclonal Antibodies
[0288] A DNA segment encoding a fusion protein comprising sequences from the human CTLA-4 and the murine CD3zeta genes was constructed by PCR amplification of cDNA clones together with bridging synthetic oligonucleotides. The encoded fusion protein contains the following sequences: i. human CTLA-4 encoding amino acids 1-190 (containing the signal peptide, the extracellular domain of human CTLA-4 and the entirety of the presumed transmembrane sequence of human CTLA-4) and ii. murine CD3zeta from amino acid 52 to the carboxy terminus (Weissman et al. (1988) Science 239: 1018-1021). The amplified PCR product was cloned into a plasmid vector and the DNA sequence was determined. The cloned insert was then subcloned into the vector pBABE (which contains a gene encoding for puromycin resistance (Morganstern, J P and Land, H Nucl. Acids Res. 18: 3587-96 (1990)) to create pBABE-huCTLA-4 / CD3z. pBAB...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tm | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com