New stapled-peptides and uses thereof
一种用途、模拟肽的技术,应用在作为炎症通路抑制剂领域,能够解决不允许阻断上游炎症通路、抗体繁重处理等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
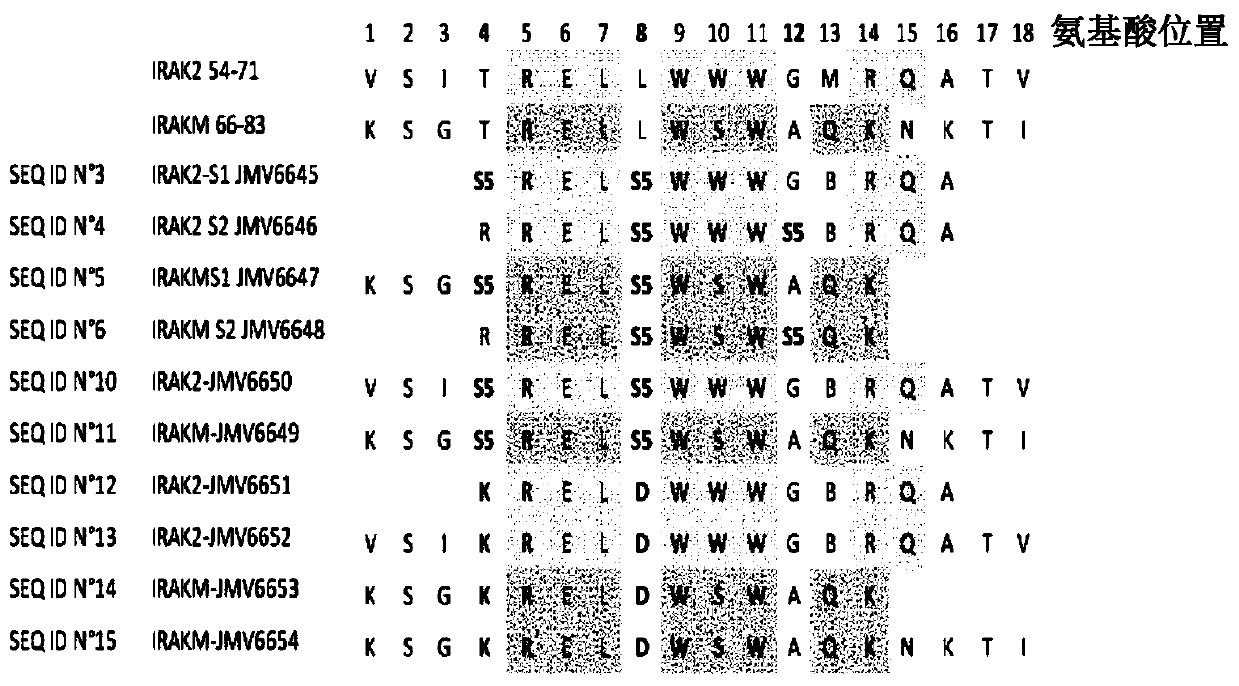
[0216] Example 1: Preparation and Characterization of Staple Peptides IRAK2 and IRAKM
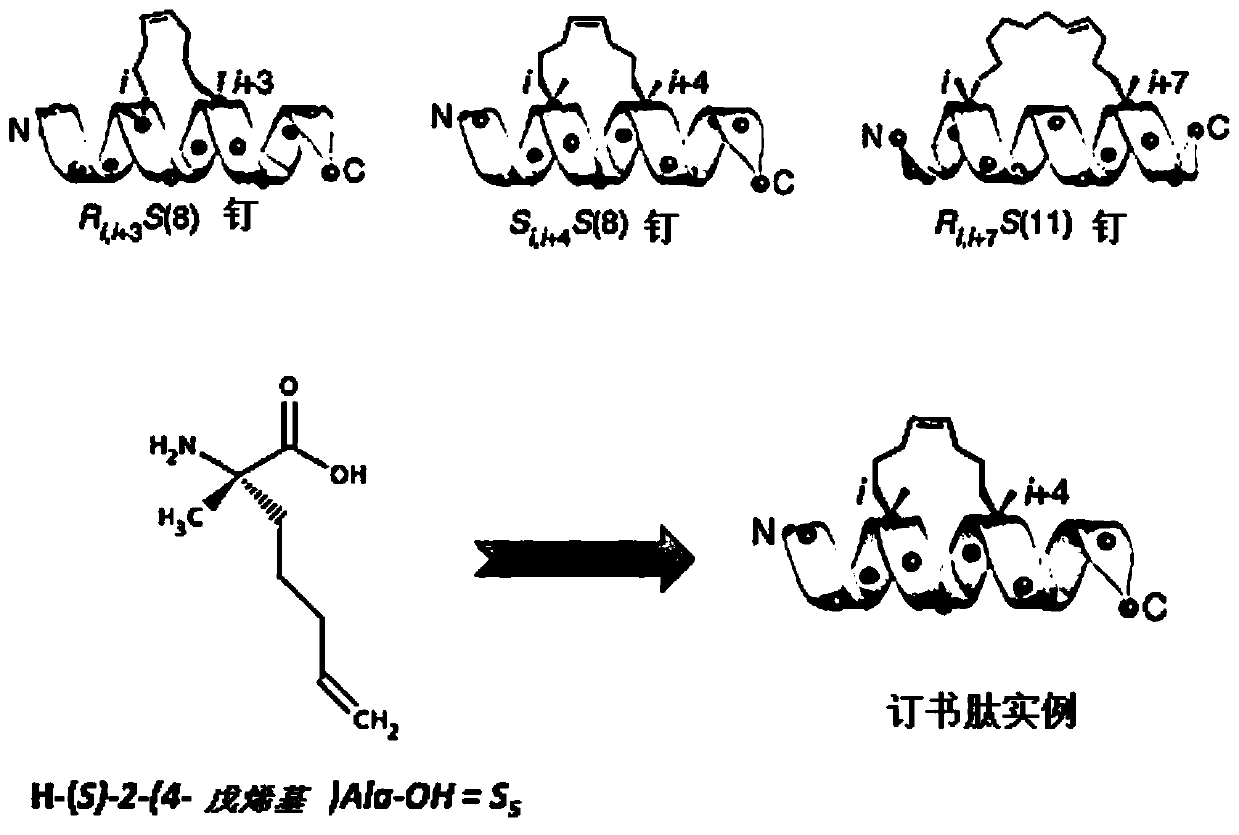
[0217] 1.1 Synthesis of stapled peptides with hydrocarbon bridges
[0218] Synthesis of IRAK2 and IRAKM stapled peptides was performed manually by the SPPS method. The synthesis scale was 0.1 mmol, and 40-RAM amphiphilic Rink amide resin was loaded at 0.4 mmol / g, with an excess of 5 equivalents for the protected amino acid. All protected amino acids and the coupling agent O-(7-azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate (HATU) were pre-dissolved in DMF to prepare a stock solution at a concentration of 0.5M. Under vortex stirring, the resin was first swelled in 6ml DMF for 15 minutes. After removal of DMF by filtration, deprotection of the Fmoc group was performed by adding a 20% piperidine / DMF solution (6 ml) and vortexing for 1 min. This is done twice. The resin was washed 3 times with DMF. Add the desired amino acid (1ml, 0.5mmol), N,N-diisopropylethyl...
Embodiment 2
[0228] Example 2: Evaluation of the specificity and efficacy of stapled peptides against the IRAK2 target
[0229] 2.1 Activation of endoplasmic reticulum stress (ER stress)
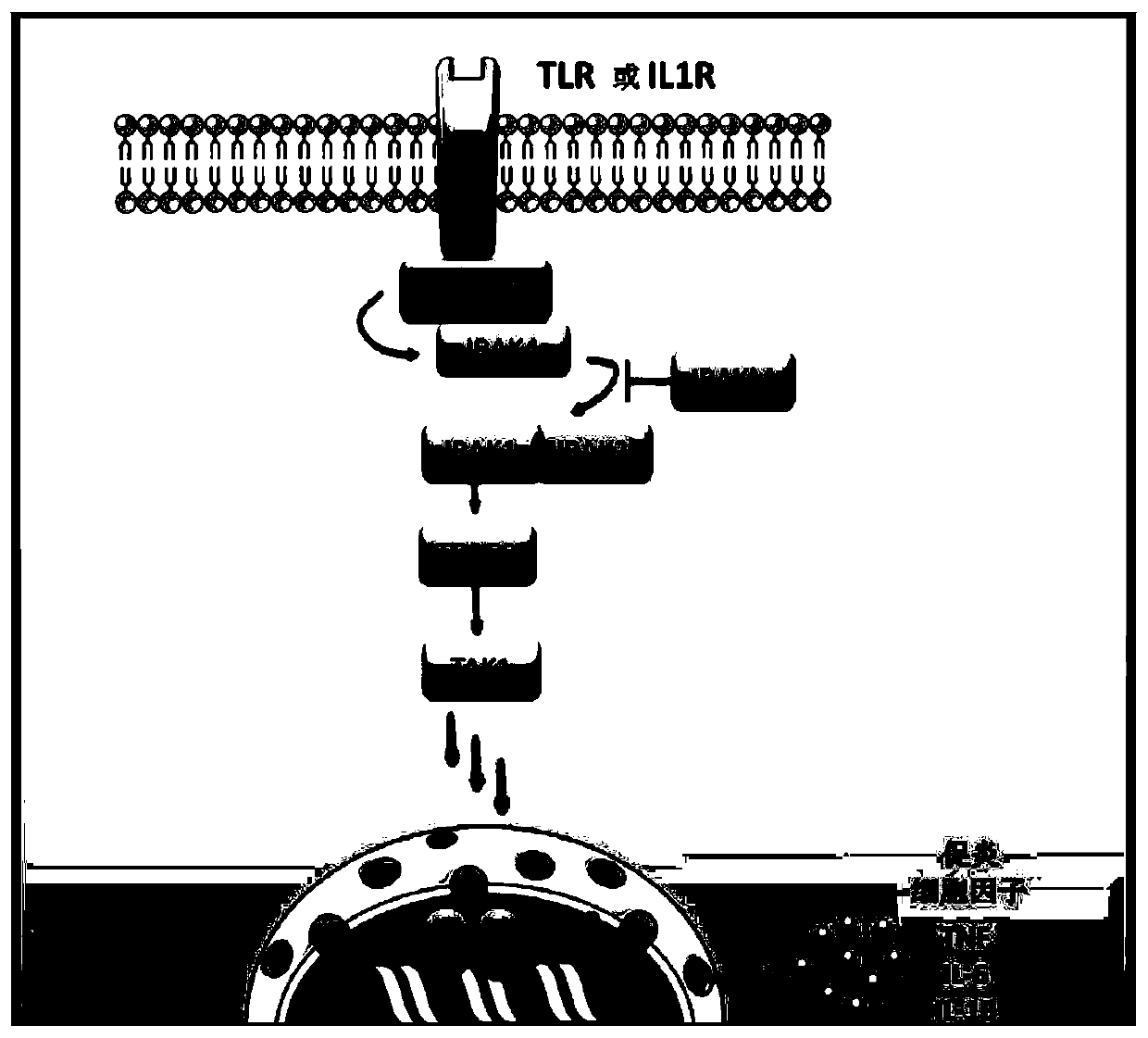
[0230] In cells, most secreted and membrane proteins are synthesized in the ER, where they are folded and assembled prior to transport. Under certain conditions, abnormal conformational proteins accumulate in the endoplasmic reticulum (ER), inducing stress (ER stress) and UPR (unfolded protein response). ER stress also contributes to the inflammatory response by differently activating the transcription of NF-κB transcription factors and inflammatory genes (pro-inflammatory cytokines). The UPR response involves activation of transcription of target genes and profound repression of translation, which increases folding and degradation capacity and limits the arrival of new proteins in the ER. Studies have shown that the IRAK2 molecule is essential for the induction of both ER stress (Benosmab et al. P...
Embodiment 3
[0243] Example 3: Evaluation of the efficacy of the stapled peptides IRAK2-S1 and IRAK-M-S1 under three different conditions: simultaneous, therapeutic sexual or prophylactic treatment
[0244] The previous examples show that staple peptide mimics the action of the natural Mydd body inhibitor IRAK-M. Indeed, it was demonstrated that exposure of THP1 monocytes to the staple peptides IRAK2-S1 and IRAKM-S1 6 h after LPS stimulation reproduced the LPS-tolerant condition, i.e. suppressed IL-6 and TNFα cytokine production. This example investigates whether different experimental setups can show better results. Three different conditions of LPS stimulation were compared: simultaneous LPS stimulation of monocytes and addition of stapled peptide (simultaneous condition: A), 6 hours before LPS stimulation (preventive condition: B) or 6 hours after ( Therapeutic conditions: C) adding staple peptide ( Figure 6 ).
[0245] For all tested conditions, THP-1 mononuclear cells (300000 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com