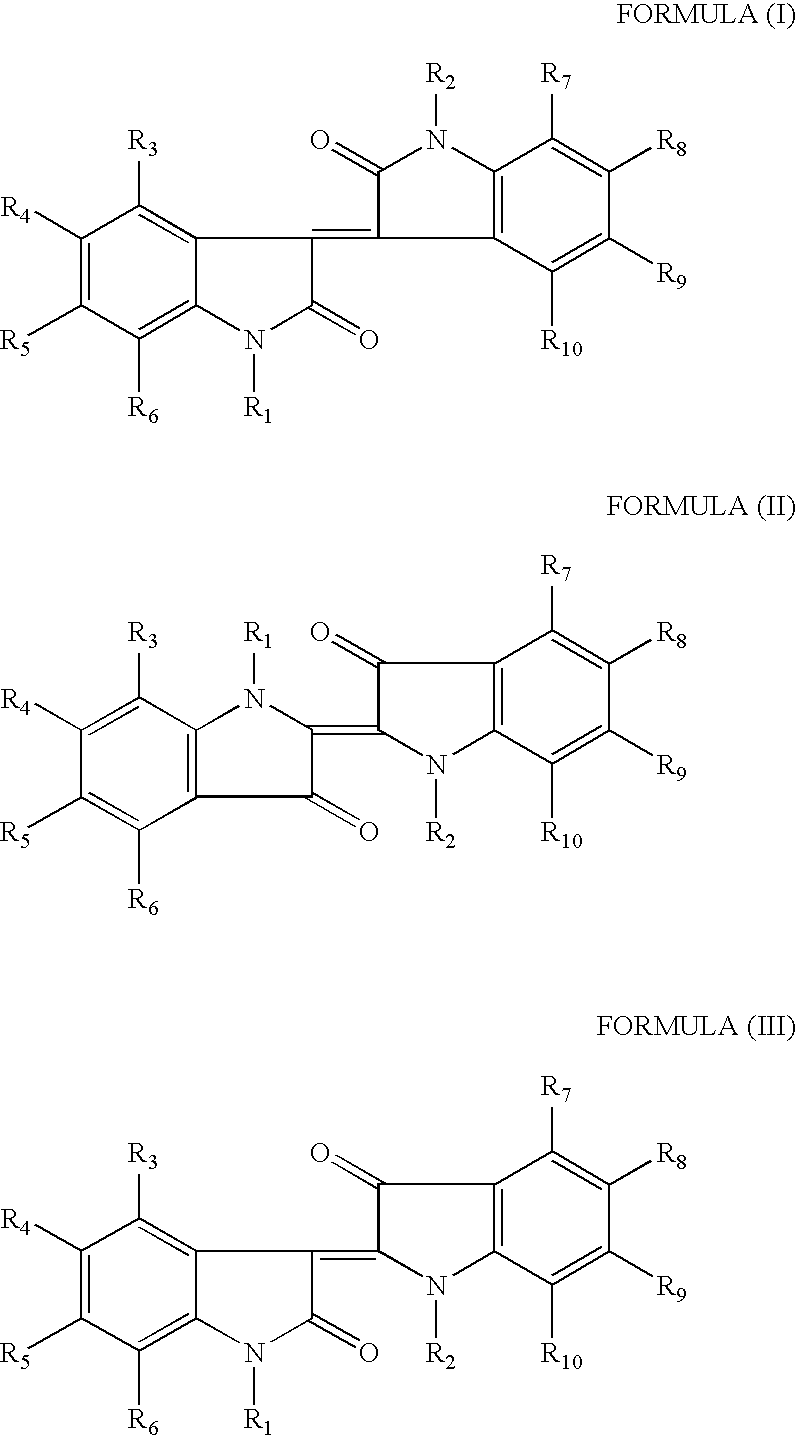
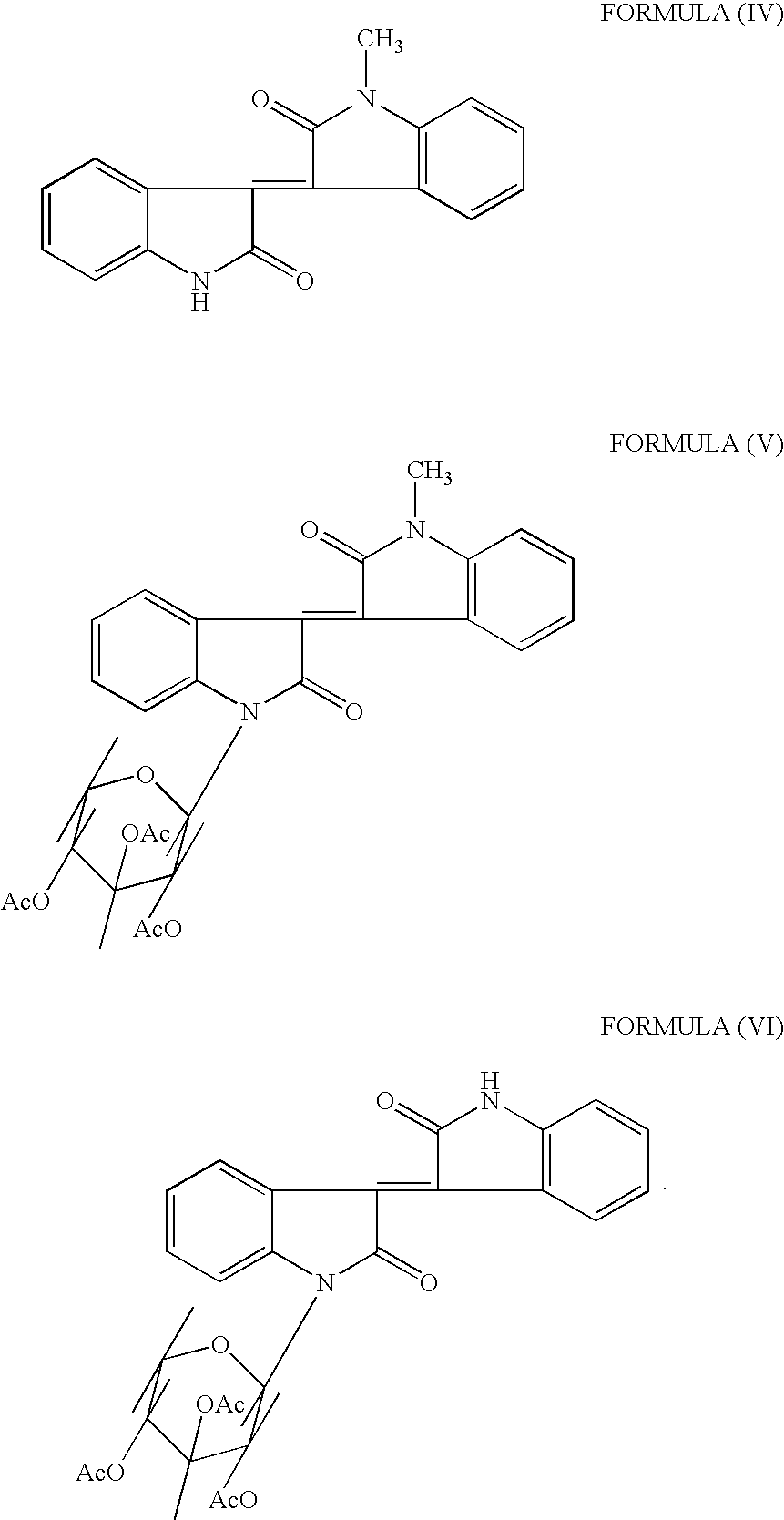
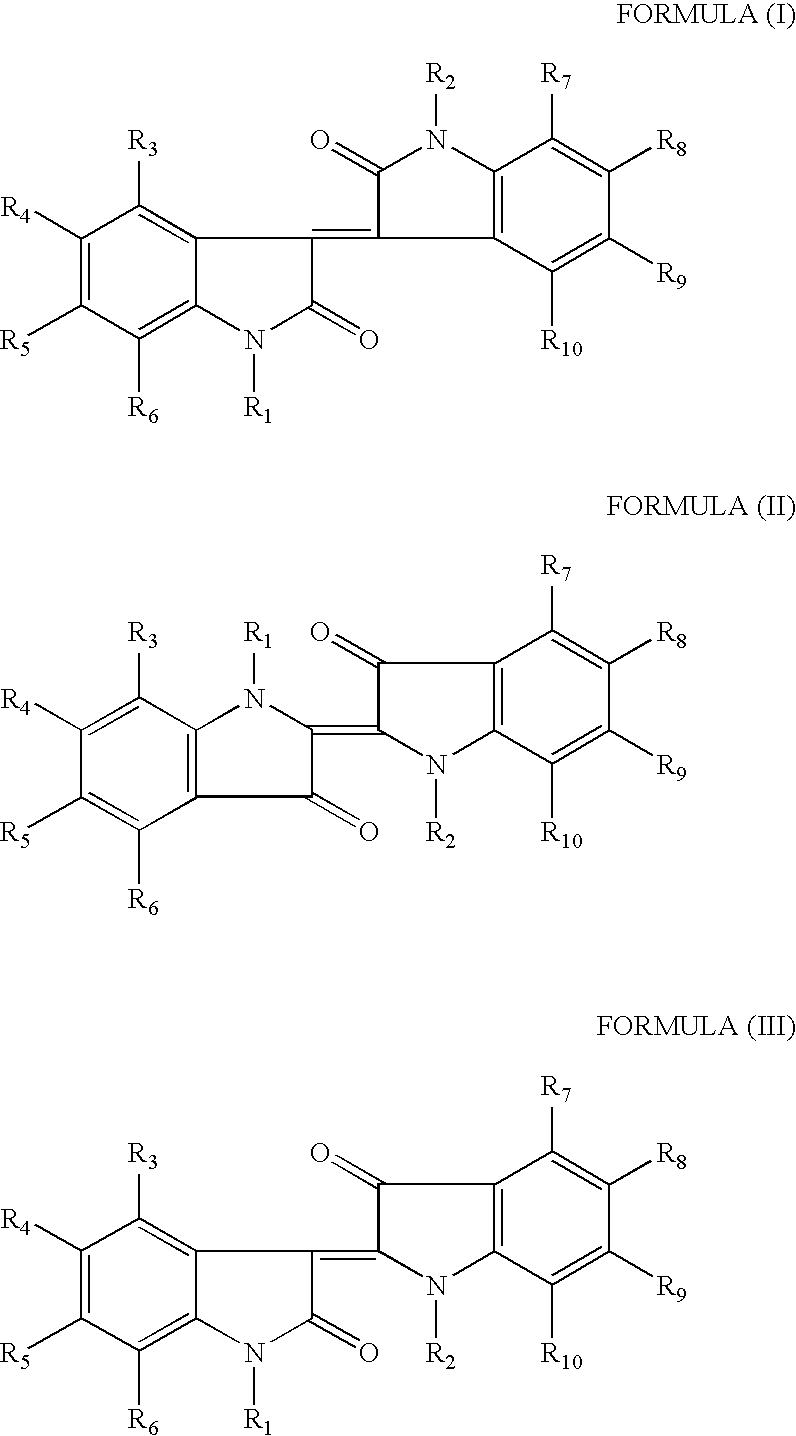
Methods of treating an inflammatory-related disease
a technology for inflammatory-related diseases and compositions, applied in drug compositions, peptides, metabolic disorders, etc., can solve the problems of inflammation or infection, life-threatening diseases in advanced stages, general unsatisfactory, etc., and achieves low side effects, low cost, and the effect of inhibiting the expression of pro-inflammatory cytokines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Meisoindigo Reduces the Secretion of IL-β in Human Monocytic Cell Line THP-1 Cells
Materials and Methods
[0121] Materials: Meisoindigo and NATURA were synthesized by Natrogen Therapeutics, Inc, purified by high performance liquid chromatography (HPLC) with a purity of 98.5%, and their structures confirmed by mass spectrometry and nuclear magnetic resonance (NMR). Meisoindigo is a dark-reddish crystal, with a molecular weight of 376. It was prepared in a solution of dimethyl sulfoxide (DMSO), and stored under −20° C. for the experiments in vitro. Human monocytic cell line, THP-1 (90), was purchased from ATCC. The cells were maintained according to the supplier's instructions. Approximately 1×105 cells / ml were cultured at 37° C., 5% CO2 for 24 hours in Modified RPMI-1640 Medium (Invitrogen) supplemented with 10% FBS.
[0122] Methods: The cells were stimulated with or without 1 μM of lipopolysaccharide (LPS, Sigma), and exposed for 24 hours to different concentrations of Meisoindigo (f...
example 2
Meisoindigo Inhibits the Secretion and Expression of IL-6 in Human Monocytic Cell Line THP-1 Cells
Materials and Methods
[0127] Materials: The representative derivative Meisoindigo was used. The cell line and the procedure of ELISA were the same as described in Example 1. Standard IL-6 protein was used to establish a standard curve for the calculation of IL-6 in the medium secreted by the cells (LPS-stimulated or non-stimulated cells in the presence or absence of Meisoindigo). A typical standard curve is shown in FIG. 3, panel A. Statistical analysis also followed the method described in Example 1.
[0128] Methods:
[0129] Real Time PCR: The effect of Meisoindigo on the transcription of IL-6 (RNA levels) was determined by a technique of real time polymerase chain reaction (real time PCR). Total RNA was extracted using a Qiagen Rneasy minit kit, and the HPRT gene was used as internal control.
[0130] Human monocytic THP-1 cells at exponential growth phase were exposed to 1 μg / ml of LPS...
example 3
Meisoindigo Suppresses the Secretion of TNF-α in Human Monocytic THP-1 Cells
Materials and Methods
[0134] The representative derivative Meisoindigo was used. The cell line and the procedure of ELISA to measure secretion of TNF-α were the same as described in Example 1, except the standard TNF-α protein was used to establish a standard curve for the calculation of the protein secreted in the medium by the cells (LPS-stimulated or non-stimulated cells in the presence or absence of Meisoindigo). A typical standard curve is shown in FIG. 4, panel A.
[0135] The effect of Meisoindigo on the transcription of TNF-α (RNA levels) was determined by a technique of real time PCR using the same procedures described in Example 2, except the specific primers for TNF-α were used as follows: 5′-TGCCCAG-ACTCGGCAAAG (SEQ ID NO. 3), and 5′GGAGAAGGGTGACCGACT (SEQ ID NO. 4). Total RNA was extracted using a Qiagen Rneasy minit kit, and the HPRT gene was used as internal control.
[0136] Human monocytic THP...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| body weights | aaaaa | aaaaa |
| neurodegenerative disorder | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com