Method for anodizing aluminum materials
anodizing aluminum and aluminum materials technology, applied in the direction of anodisation, surface reaction electrolytic coating, electrolytic coating, etc., can solve the problems of not being able to keep using this method in the future, not being able to achieve all three characteristics at the same time, and having rather undesirable side effects. , serious drawbacks, etc., to achieve the effect of low porosity, high corrosion resistance and substantial porosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
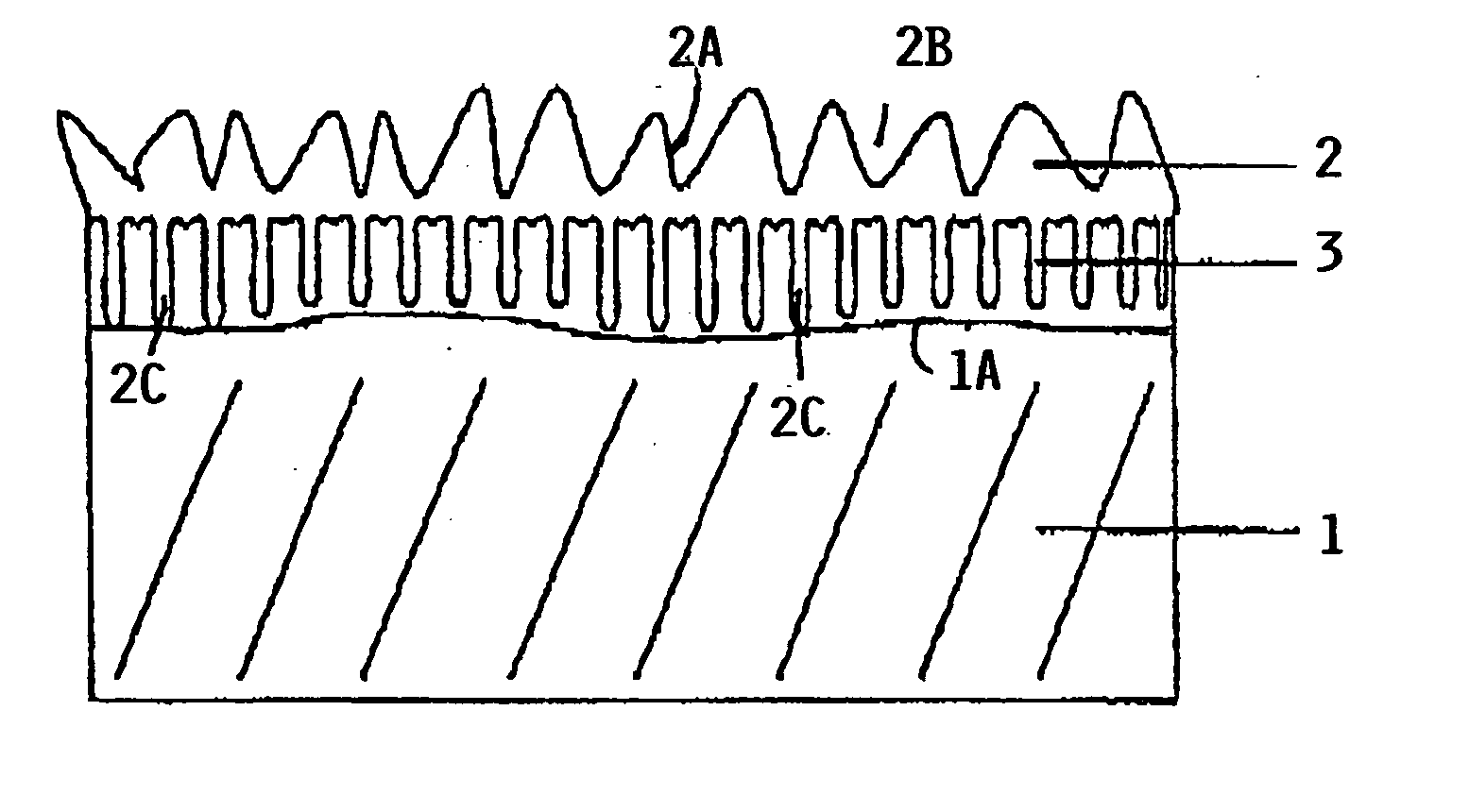
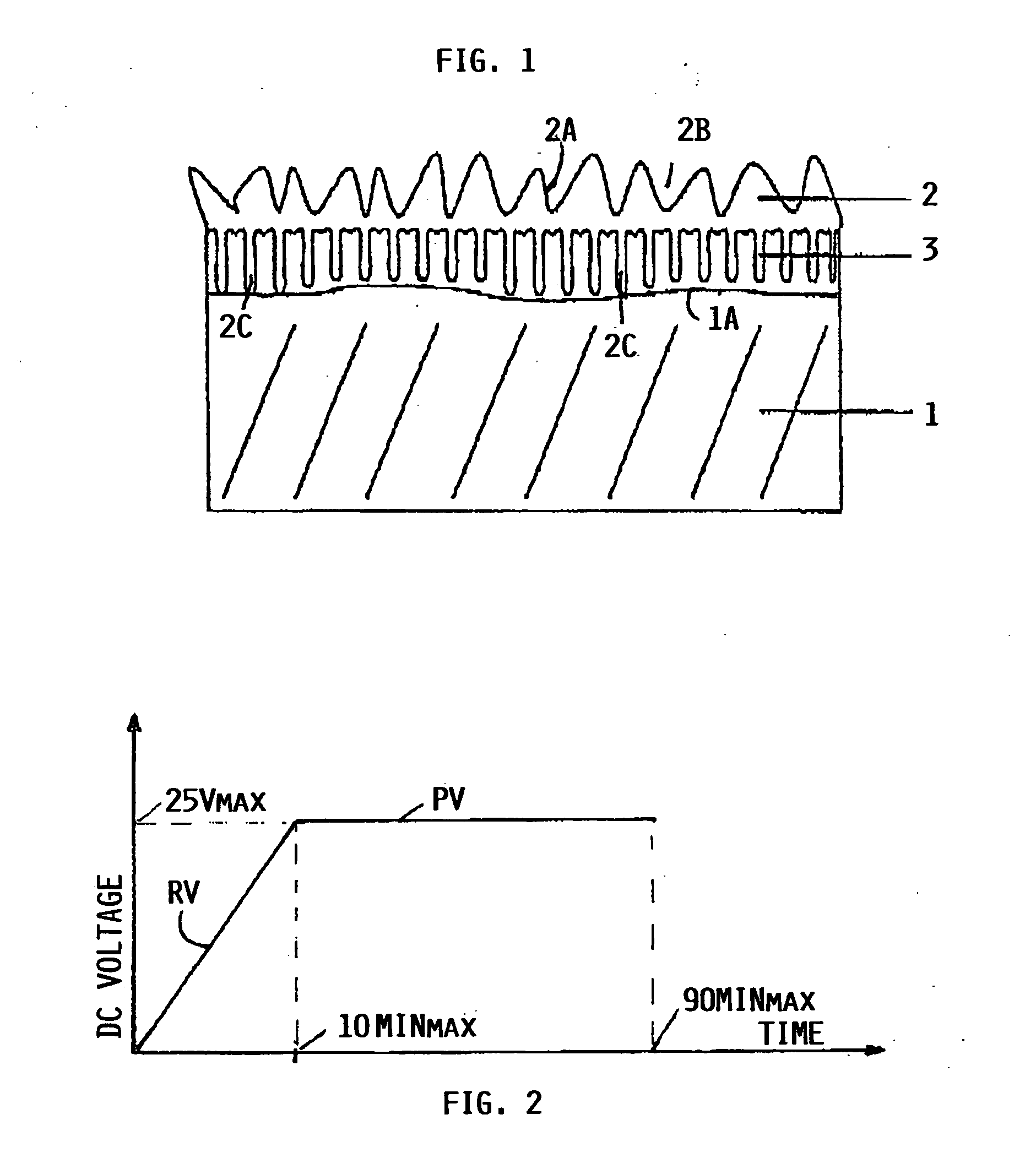
[0030]FIG. 1 shows an aluminum component 1 provided with a first anodized layer 2 and a second anodized layer 3. The layer thickness is exaggerated having regard to the fact that the thickness of the layers is within the range of a few microns, preferably in the range of 1 to 10 μm as mentioned above.
[0031] It will be noted that in FIG. 1 the anodized layer 2, which is produced first, appears as top layer having a very rugged surface 2A with deep pores 2B and roots 2C reaching into the second layer 3 shown to adhere to the surface 1A of the component 1. The second layer 3 is anodized after the first layer 2 has been anodized. Yet, the second layer 3 appears between the first layer 2 and the surface 1A. It is assumed that the second anodized layer 3 can grow through the deep pores 2B and then adhere to the surface 1A and to the first layer 2 due to affinities between the aluminum materials on the one hand and the two different electrolytes on the other hand. In this context it is po...
PUM
| Property | Measurement | Unit |
|---|---|---|
| DC voltage | aaaaa | aaaaa |
| DC voltage | aaaaa | aaaaa |
| DC voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com