Tryparedoxin, expression plasmid, process of preparation, method of use, test kit and pharmaceutical composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Determination of Tryparedoxin Activity
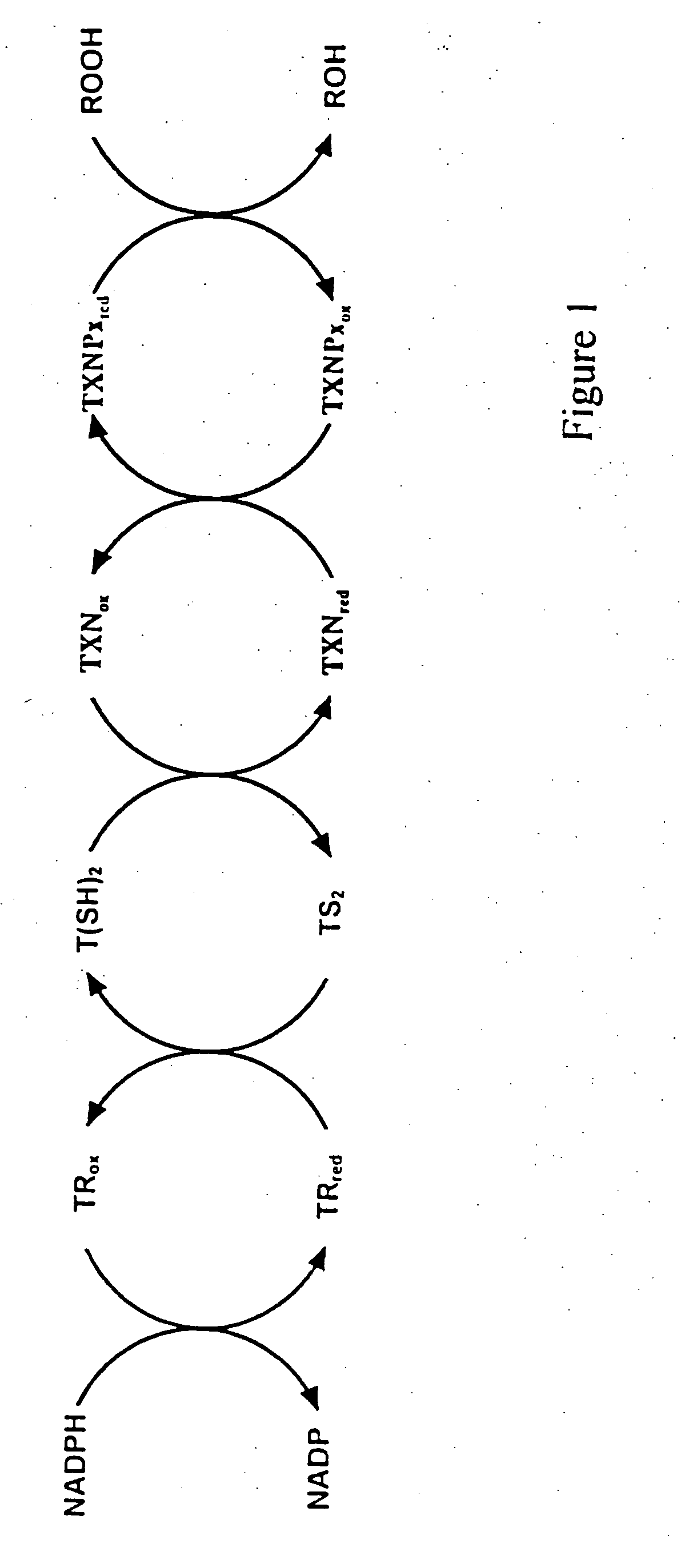
[0043] In essence, the activity of tryparedoxin activity is measured by coupling the catalytic reduction of hydroperoxide mediated by tryparedoxin peroxidase to NADPH consumption by means of trypanothione and trypanothione reductase. For example, an assay sample may contain 0.1 mM NADPH in 50 mM Hepes pH 7.6, 1 mM EDTA, 50 M H2O2 or t-butyl hydroperoxide (t-bOOH), 45 M T(SH)2, 16.5 μg / ml tryparedoxin peroxidase and 0.34 U trypanothione reductase and an unknown amount of tryparedoxin. Unless otherwise stated, the reaction is started with the addition of the hydroperoxide. Dihydro-trypanothione is obtained by chemical reduction of TS2 (Bachem, Switzerland) as described (Fairlamb et al., 1986). t-BOOH may be replaced by other hydroperoxides, such as H2O2, linoleic acid hydroperoxide or phosphatidylcholine hydroperoxide.
[0044]FIG. 6 demonstrates that trypanothione reductase, T(SH)2, tryparedoxin and tryparedoxin peroxidase are indispensable for th...
example 3
Characterisation of Tryparedoxin I by Partial Proteins Sequencing
[0045] Since the N-terminus of tryparedoxin I was blocked, the protein was digested with either bovine trypsin or endoproteinase Glu-C from Staphylococcus aureus (both sequencing grade, Promega) according to Stone and Williams (1993). The peptides were separated by HPLC (Applied Biosystems 172A) on an Aquapore OD-300 RP-18 column. Automated Edman degradation was performed with an Applied Biosystems, Inc. sequencer with an on-line C-18 reverse phase HPLC. Database searches were performed with the BLAST and FASTA programs. Peptides were aligned with the Bestfit program, Genetics Computer Group (GCG), Madison, Wis., USA.
[0046] Seven fragments could be sequenced and could be aligned to a thioredoxin-like protein of C. elegans (FIG. 7).
example 4
Use of Sequenced Fragments of Tryparedoxin I to Elucidate the Encoding DNA
[0047] Cells culture and DNA extraction: C. fasciculata (HS6) was grown as described by Shim and Fairlamb (1988). The cells were harvested by centrifugation for 15 min at 7000 rpm, washed twice with saline solution (0.9% NaCl) and resuspended in 5 ml buffer (50 mM TrisHCl, 100 mM EDTA, 15 mM NaCl, 0.5% SDS, 100 μg ml−1 Proteinase K, pH 8.0). Resuspended cells were preincubated at 50° C. for 40 min. The genomic DNA was extracted twice with equivalent volumes of phenol (incubation: 60° C. for 45 min; centrifugation: 20 min, 4500 rpm) followed by phenol:chloroform:isoamyl alcohol (25:24:1) and chloroform:isoamyl alcohol extraction (24:1). Genomic DNA was precipitated with sodium acetate and ethanol.
[0048] Primers, hybridization probes and sequence analysis: Based on the peptide sequences of tryparedoxin I (Nogoceke et al., 1997) degenerate oligodeoxyribonucleotides were synthesized. Polymerase chain reaction (P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Atomic weight | aaaaa | aaaaa |
| Atomic weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com