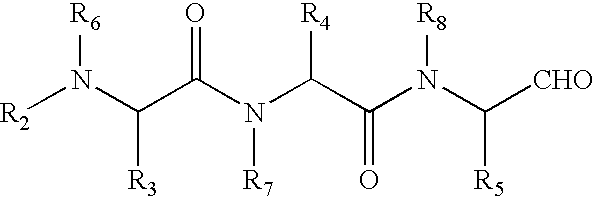
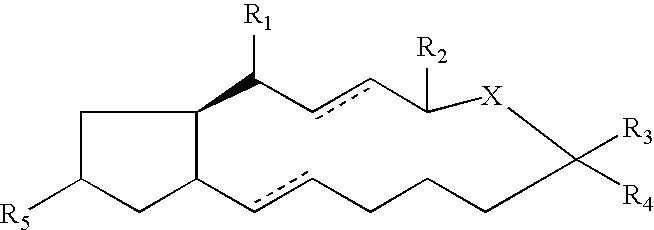
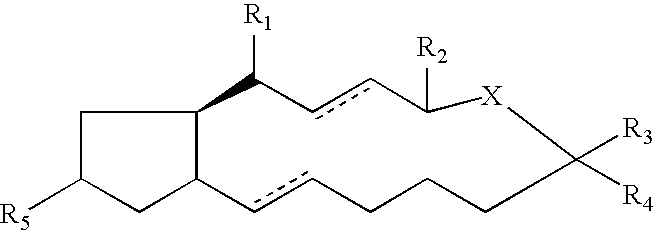
Compounds and methods for pharmico-gene therapy of epithelial sodium channel associated disorders
a technology pharmico-gene therapy, which is applied in the field of compound and method for pharmico-gene therapy of epithelial sodium channel associated disorders, can solve the problems of limited packaging capacity of this vector, mediated gene delivery of cftr, and inability to achieve optimal transduction of airway cells as measured by vector derived cftr mrna, etc., to achieve the effect of improving the functional conversion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Endosomal Processing Limits AAV Transduction
[0166] Based on the finding that basolateral membranes have higher endocytic rates and UV irradiation enhances endosomal uptake and rAAV transduction from the apical membrane, it is possible that endosomal pathways influencing viral uptake and transport to the nucleus may be limiting from the apical membrane. In contrast, these pathways may be active at maximal levels from the basolateral membrane of airway epithelial cells. To further investigate the importance of endosomal processing, the effect(s) of several chemical agents known to alter endosomal processing was evaluated.
Methods
[0167] Initial studies were performed in confluent primary human fibroblasts since dose titrations and toxicity could be quickly assessed. Selected agents were used to treat fibroblast monolayers prior to rAAV infection. rAAV transduction was assessed at 96 hours post-infection by FACS analysis, and the percentage of dead cells was simultaneously assessed b...
example 2
Endosomal Processing Inhibitors May Increase rAAV Transduction in Polarized Airway Cells
Materials and Methods
[0172] Primary culture of human bronchial epithelia and reagents utilized. Primary human airway epithelial cells were collected by enzymatic digestion of bronchial samples from lung transplants, as previously described (Kondo et al., 1991; Zabner et al., 1996). Isolated primary airway cells were seeded at a density of 5×105 cells / cm2 onto collagen-coated Millicell-HA culture inserts (Millipore Corp., Bedford, Mass.). Primary cultures were grown at the air-liquid interface for more than 2 weeks, by which time differentiation into a mucociliary epithelium occurs. The culture medium, used to feed only the basolateral side of the cells, contained 49% DMEM, 49% Ham's F12 and 2% Ultraser G (BioSepra, Cedex, France). Dimethyl Sulphoxide (DMSO), camptothecin (Camp), etoposide (Etop), aphidicolin (Aphi), hydroxyurea (HU) and genistein (Geni) were purchased from Sigma (St. Louis, Mo...
example 3
Expression of the LacZ Gene in Lung
Airway Epithelium and Liver In vivo
[0201] The in vivo activity of rAAV in the presence or absence of an agent of the invention in the lung or liver may be tested using the LacZ gene. A rAAV vector containing the LacZ gene, recombinant AV.LacZ (5×1010 particles), was administered to mouse lung either as virus alone in PBS or virus in combination with 40 μM LLnL in PBS. Virus was directly instilled into C57Balb / c mice trachea with a 30 G needle in a total volume of 30 μl. To insure the spread of the virus in mouse lung, 50 μl air was pumped into lung through the same syringe immediately after virus was administrated. Ninety days after infection, lungs were harvested intact and fixed in 4% paraformaldehyde followed by cryosection. AAV-mediated transgene expression was evaluated by 10 μm tissue sections staining forLac Z.
[0202] Recombinant AV.LacZ (5×1010 particles) was also administered to mouse liver either as virus alone in PBS, virus in combinat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com