Treatment of pompe's disease
a pompe's disease and patient technology, applied in the field of pompe's disease treatment, can solve the problems of pompe's disease experiments that were not successful, progressive muscular weakness and cardiac failure, and damage to the accumulation of metabolites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Transgenes
[0079] (a) cDNA construct
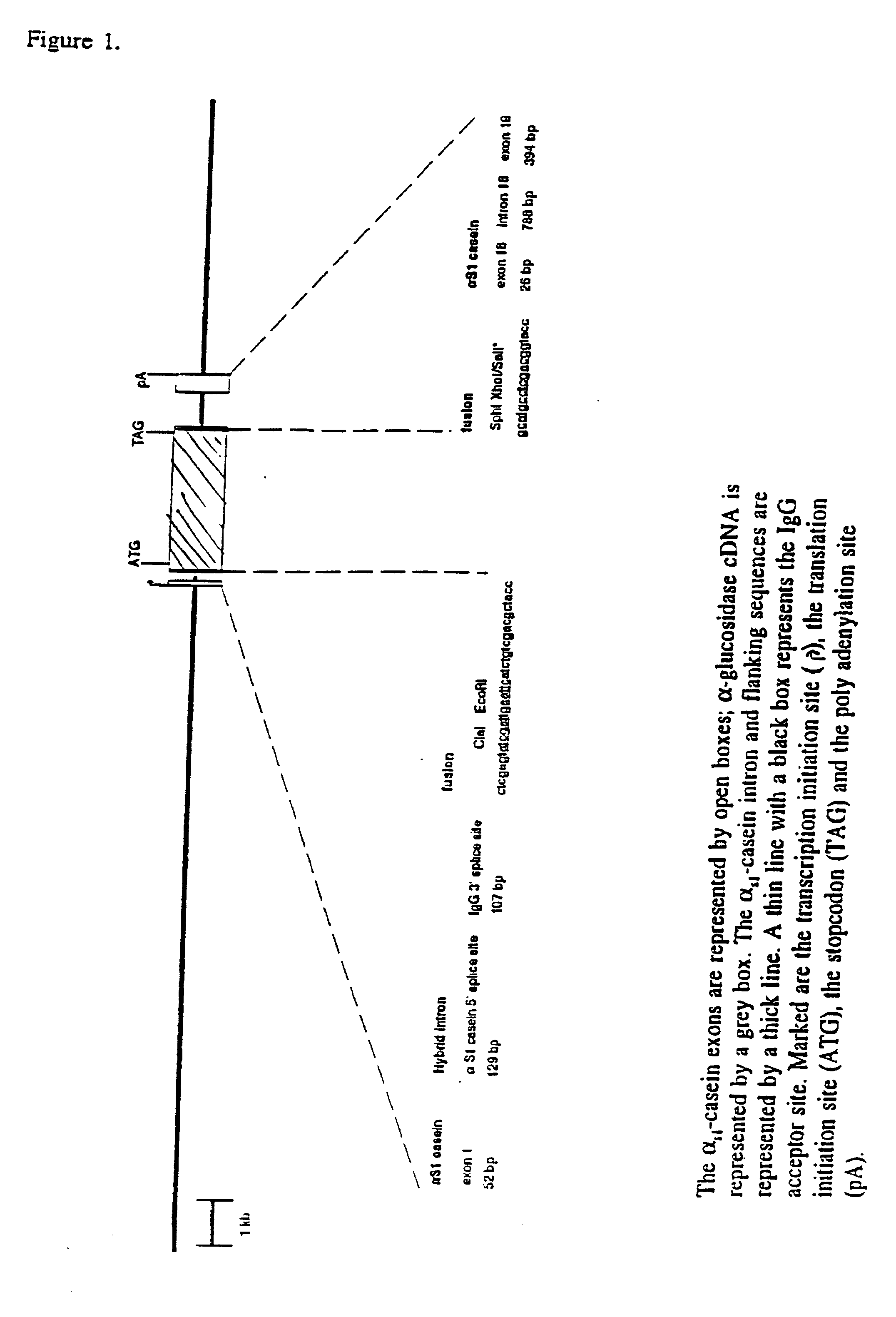
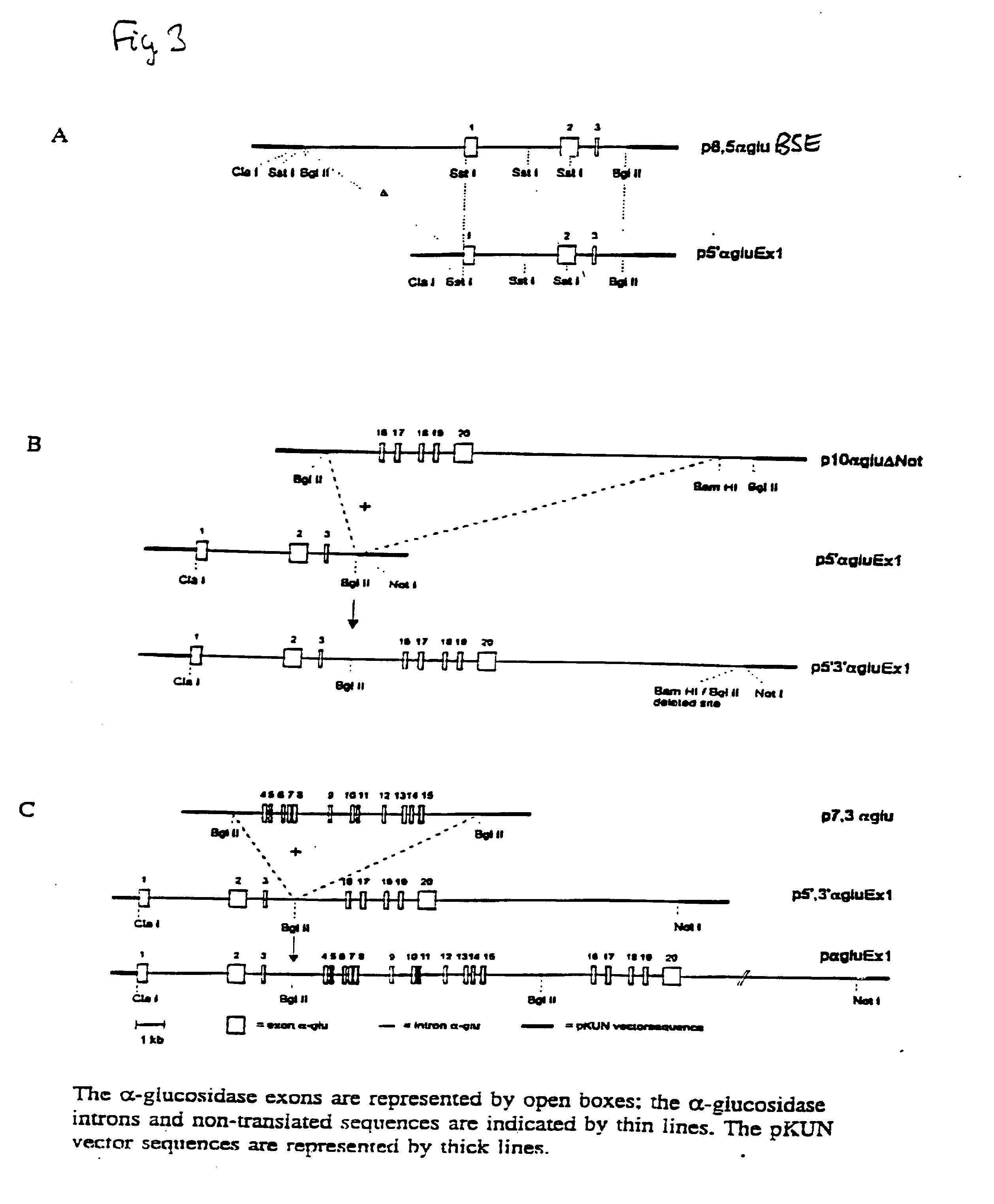
[0080] Construction of an expression vector containing cDNA encoding human acid .alpha.-glucosidase started with the plasmid p16,8hlf3 (see DeBoer et al. (1991) & (1993), supra) This plasmid includes bovine .alpha.S1-casein regulatory sequences. The lactoferrin cDNA insert of the parent plasmid was exchanged for the human acid .alpha.-glucosidase cDNA (Hoefsloot et al. EMBO J. 7,1697-1704 (1988)) at the ClaI site and SalI site of the expression cassette as shown in FIG. 1. To obtain the compatible restriction sites at the ends of the .alpha.-glucosidase cDNA fragment, plasmid pSHAG2 (id.) containing the complete cDNA encoding human .alpha.-glucosidase was digested with EcoRI and SphI and the 3.3 kb cDNA-fragment was subcloned in pKUN7.DELTA.C a pKUN1 derivative (Konings et al., Gene 46, 269-276 (1986)), with a destroyed ClaI site within the vector nucleotide sequences and with a newly designed polylinker: HindIII ClaI EcoRI SphI Xho...
example 2
[0099] The cDNA and genomic constructs were linearized with NotI and injected in the pronucleus of fertilized mouse oocytes which were then implanted in the uterus of pseudopregnant mouse foster mothers. The offspring were analyzed for the insertion of the human .alpha.-glucosidase cDNA or genomic DNA gene construct by Southern blotting of DNA isolated from clipped tails. Transgenic mice were selected and bred.
[0100] The genomic constructs linearized with NotI were also injected into the pronucleus of fertilized rabbit oocytes, which were implanted in the uterus of pseudopregnant rabbit foster mothers. The offspring were analyzed for the insertion of the alpha-glucosidase DNA by Southern blotting. Transgenic rabbits were selected and bred.
example 3
Analysis of Acid .alpha.-Glucosidase in the Milk of Transgenic Mice
[0101] Milk from transgenic mice and nontransgenic controls was analyzed by Western Blotting. The probe was mouse antibody specific for human acid .alpha.-glucosidase (i.e., does not bind to the mouse enzyme). Transgenes 1672 and 1673 showed expression of human acid .alpha.-glucosidase in milk (FIG. 4). Major and minor bands at 100-110 kD and 76 kD were observed as expected for .alpha.-glucosidase. In milk from non-transgenic mice, no bands were observed.
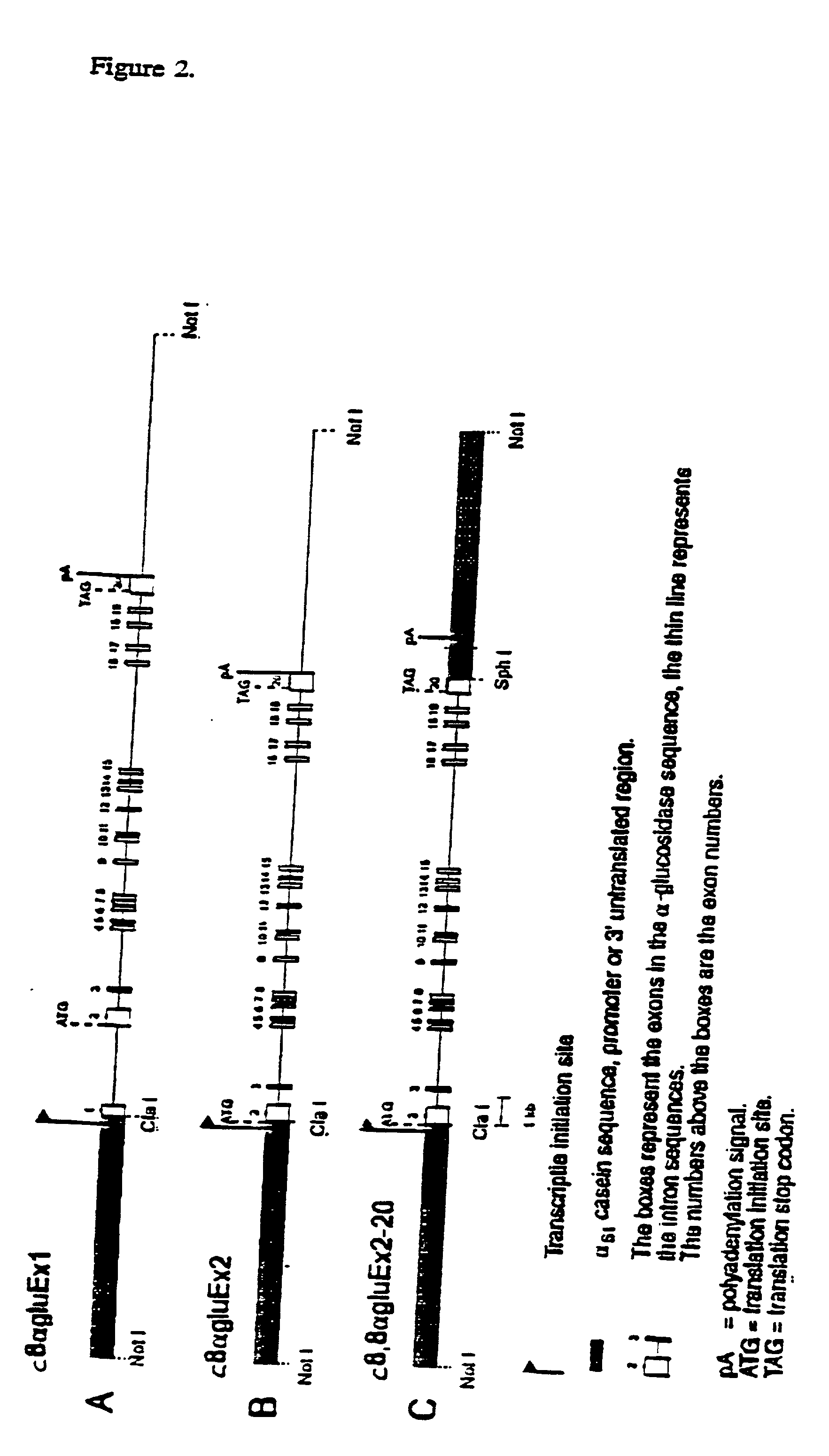
[0102] The activity of human acid .alpha.-glucosidase was measured with the artificial substrate 4-methylumbelliferyl-.alpha.-D-glucopyranoside in the milk of transgenic mouse lines (See Galiaard, Genetic Metabolic Disease, Early Diagnosis and Prenatal Analysis, Elsevier / North Holland, Amsterdam, pp. 809-827 (1980)). Mice containing the cDNA construct (FIG. 1) varied from 0.2 to 2 .mu.mol / ml per hr. The mouse lines containing the genomic construct (FIG. 2, panel A) e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| bed height | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com