Dosage form comprising means for changing drug delivery shape
a technology of drug delivery and shape, applied in the direction of osmotic delivery, pill delivery, medical preparations, etc., can solve the problems of many patients' difficulty in delivering therapeutic agents for their intended therapy, limited use, and difficult to control the dose of therapeutic agents, so as to avoid and/or reduce the risks of dose dumping and maintain the effect of physical integrity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
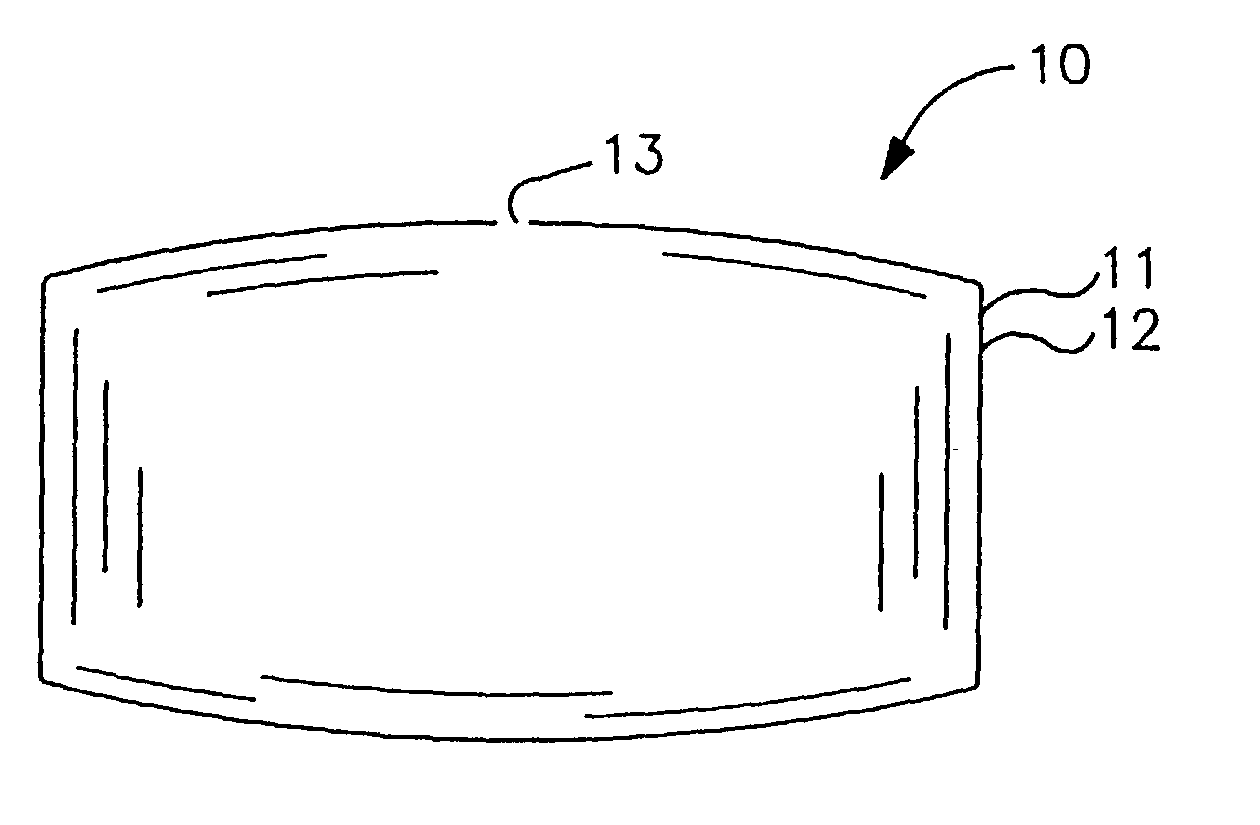
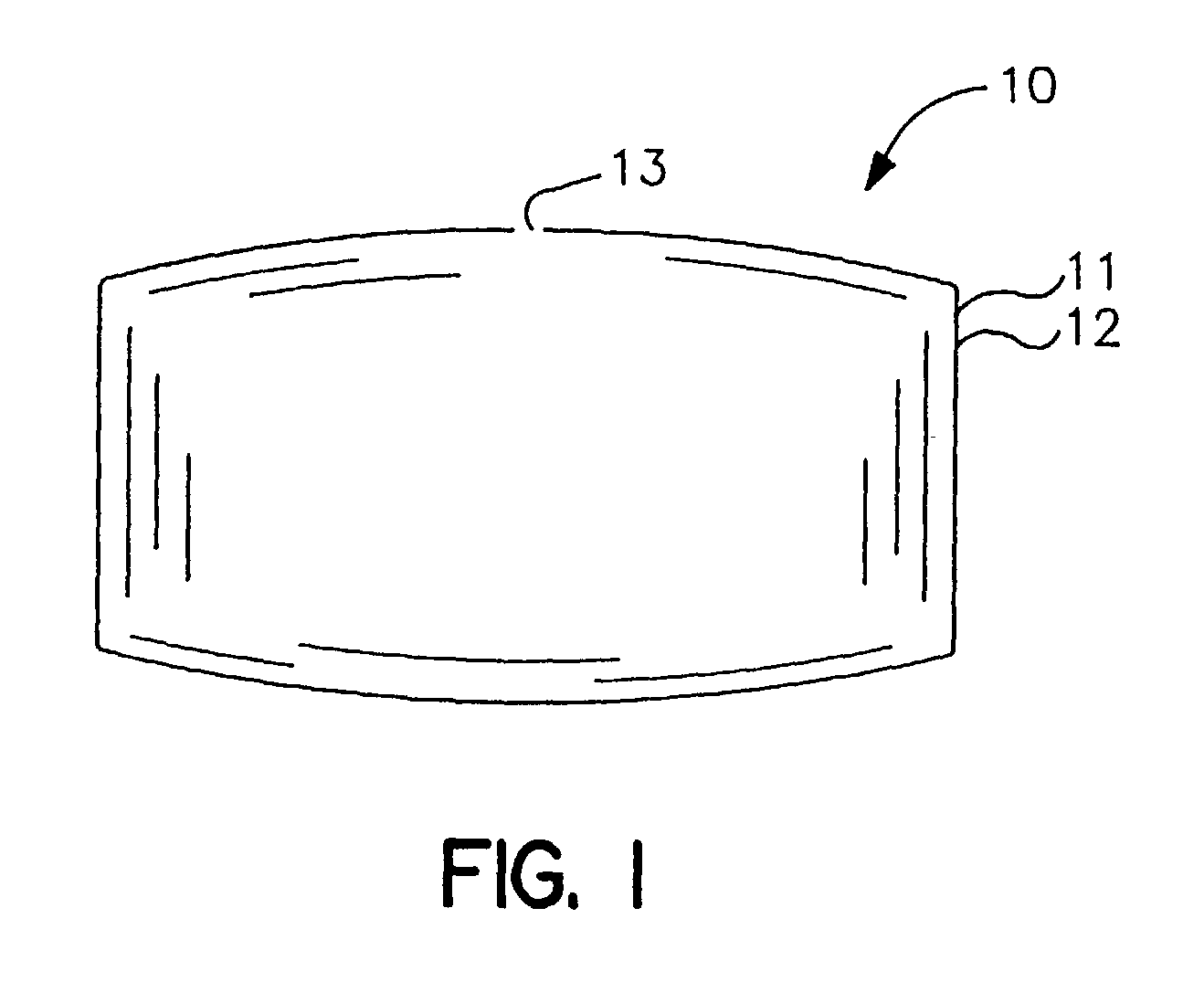
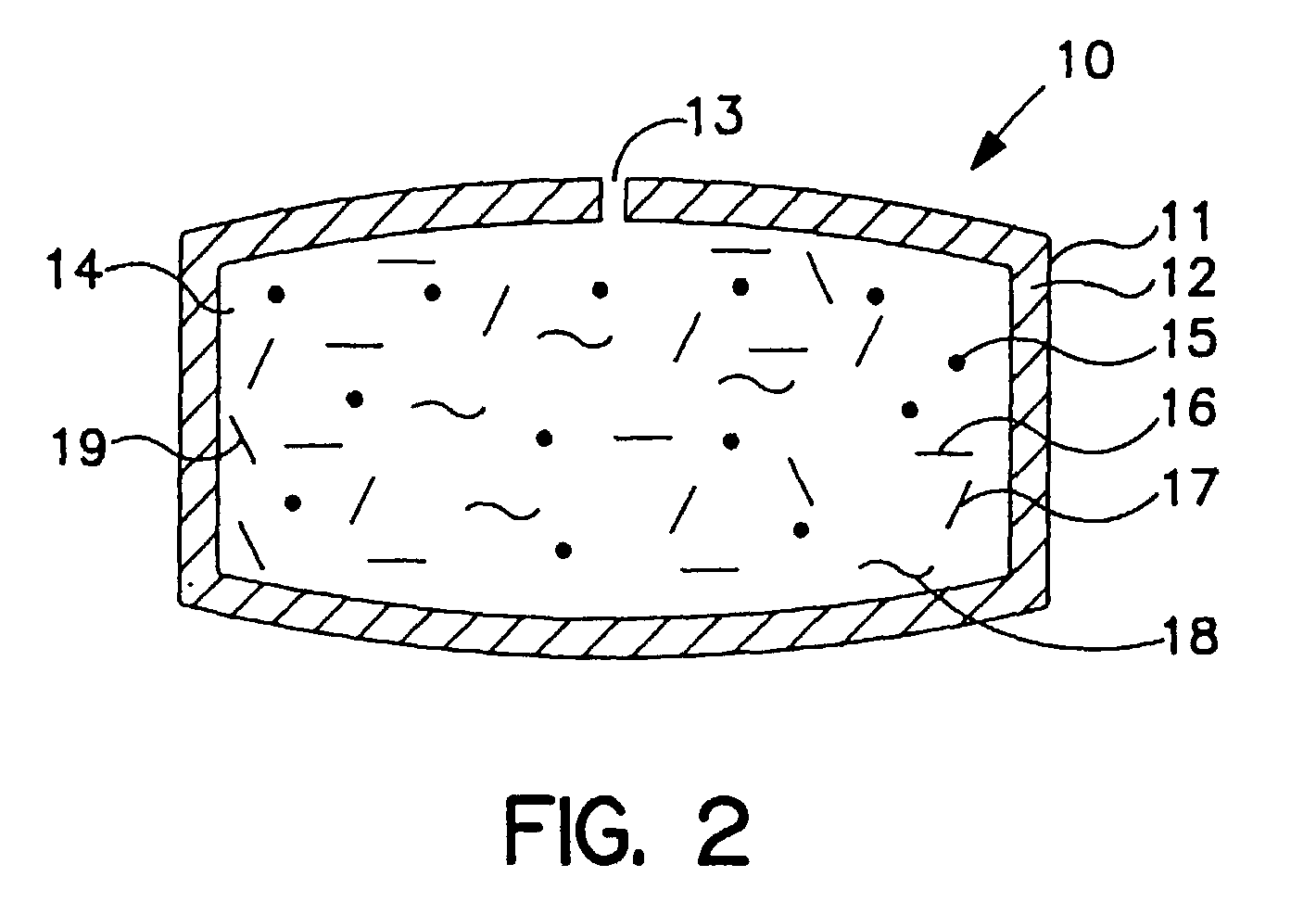
[0058] An osmotic dosage form designed to deliver at controlled rate the calcium-channel blocker, nifedipine, for once a day treatment of angina and hypertension is fabricated according to this invention. The dosage form consists of a layered tablet which is coated with the specialized rate-controlling membrane that changes shape as it functions. The layer of the tablet consists of the active drug and other layer forming ingredients.
[0059] The drug layer of the dosage form is formulated by first micronizing 200 grams of the drug to a particle size of approximately 3-5 microns. Then, 745 grams of polyethylene oxide having a molecular weight of approximately 200,000 grams per mole, and 50 grams of hydroxpropylmethylcellulose having a hydroxypropyl content of 10 weight percent, a methoxyl content of 29 weight percent, and a molecular weight of 11,300 grams per mole, are passed through a sieve having 40 wires per inch. All the components are then dry mixed. To the mixture is added ethyl...
example 2
[0062] The above procedure is repeated, except in this example the drug is a member selected from the group consisting of doxazosin, ebastine, fludarbine, formoterol, letrozle, lodoxamide moexipril, penciclovir, sertaline, sparfloxacin, and spirapril.
example 3
[0063] An osmotic dosage form designed to deliver at controlled rate the calcium-channel blocker, nifedipine, for once a day treatment of angina and hypertension is fabricated according to this invention. The dosage form consists of a two layer tablet which is coated with the invention's specialized rate-controlling membrane which changes shape as it functions. One layer of the tablet consists of the active drug and the other layer of the tablet consists of a push layer.
[0064] The drug layer is formulated by first by micronizing 200 grams of the drug to a particle size of approximately 3-5 microns. Then, 745 grams of polyethylene oxide having a molecular weight of approximately 200,000 grams per mole, and 50 grams of hydroxypropylmethylcellulose having a hydroxypropyl content of 10 weight percent, a methoxyl content of 29 weight percent, and a molecular weight of 11,300 grams per mole, are passed through a sieve having 40 wires per inch. All the components are then dry mixed. To the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com