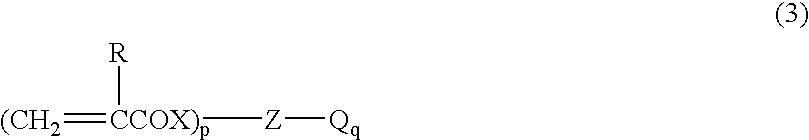Polyurethane foam, process for producing the same, and foam forming composition
a polyurethane foam and polyurethane technology, applied in the field of polyurethane foam, can solve the problems of easy non-uniform cell formation, decreased expansion ratio of the obtained foam, and unsatisfactory cross-linking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
production example 1
Production of Glycerol Monoacrylate
[0176] Using 0.3 g (0.003 mole) of sulfuric acid as a catalyst, 72 g (1 mole) of acrylic acid was reacted with 92 g (1 mole) of glycerol. The reaction mixture was neutralized with 0.34 g (0.006 mole) of potassium hydroxide, and 0.15 g (0.1 mass %) of hydroquinon was added as a stabilizer. Thus, glycerol monoacrylate (A1-1) was obtained.
production example 2
Production of Glycerol Diacrylate
[0177] Using 0.6 g (0.006 mole) of sulfuric acid as a catalyst, 144 g (2 moles) of acrylic acid was reacted with 92 g (1 mole) of glycerol. The reaction mixture was neutralized with 0.68 g (0.012 mole) of potassium hydroxide, and 0.20 g (0.1 mass %) of hydroquinon was added as a stabilizer. Thus, glycerol diacrylate (A1-2) was obtained.
production example 3
Production of Pentaerythritol PO 4-molar Adduct Triacrylate)
[0178] Using 0.9 g (0.009 mole) of sulfuric acid as a catalyst, 216 g (3 moles) of acrylic acid was reacted with 368 g (1 mole) of a polyol (hydroxyl value of 610) in which 4 moles of PO was added to 1 mole of pentaerythritol. The reaction mixture was neutralized with 1.01 g (0.018 mole) of potassium hydroxide, and 0.53 g (0.1 mass %) of hydroquinon was added as a stabilizer. Thus, pentaerythritol PO 4-molar adduct triacrylate (A1-3) was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| mass % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com