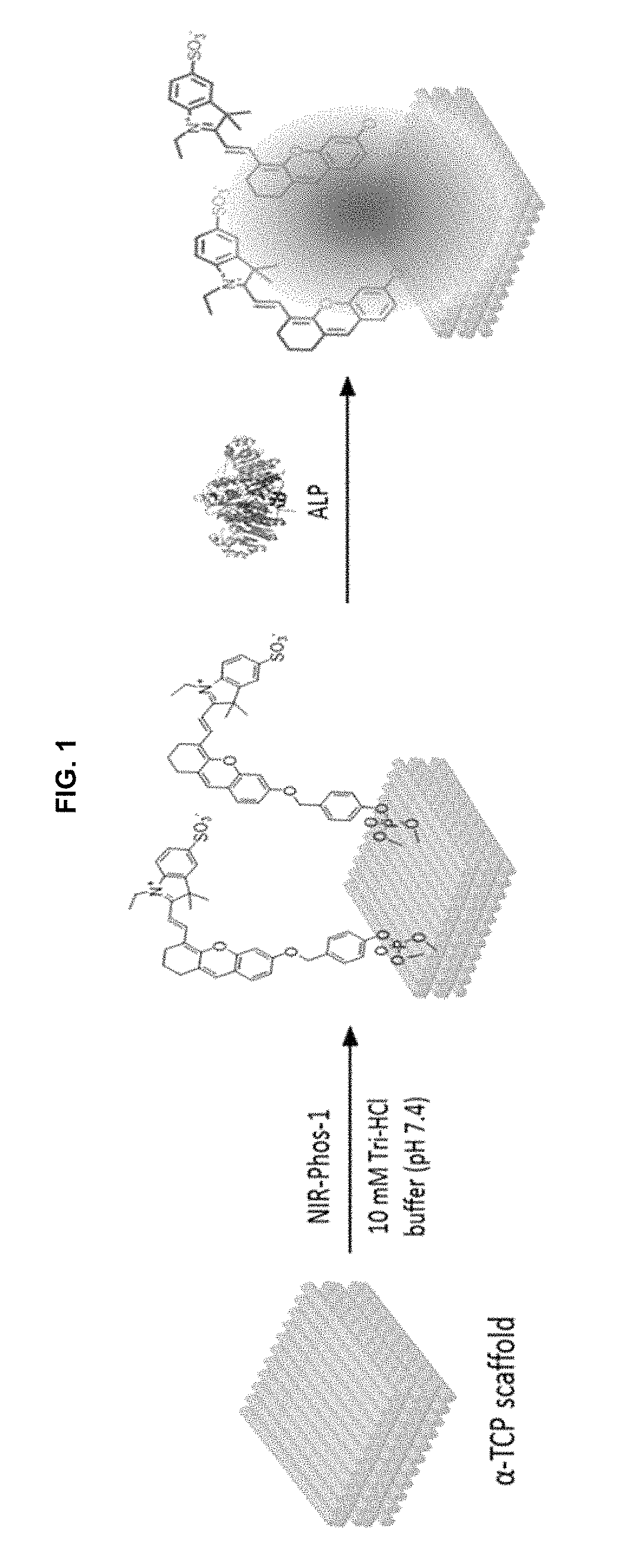
Near-infrared fluorescent probe for detecting alkaline phosphatase and manufacturing method thereof
a fluorescent probe and alkaline phosphatase technology, applied in the field of near-infrared fluorescent probes, can solve the problems of unresolved detailed mechanisms of alp activity regulation during pathogenesis, diverse physiological and pathological functions of alps, etc., and achieve the effect of selectively detecting alp only quickly and accurately
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
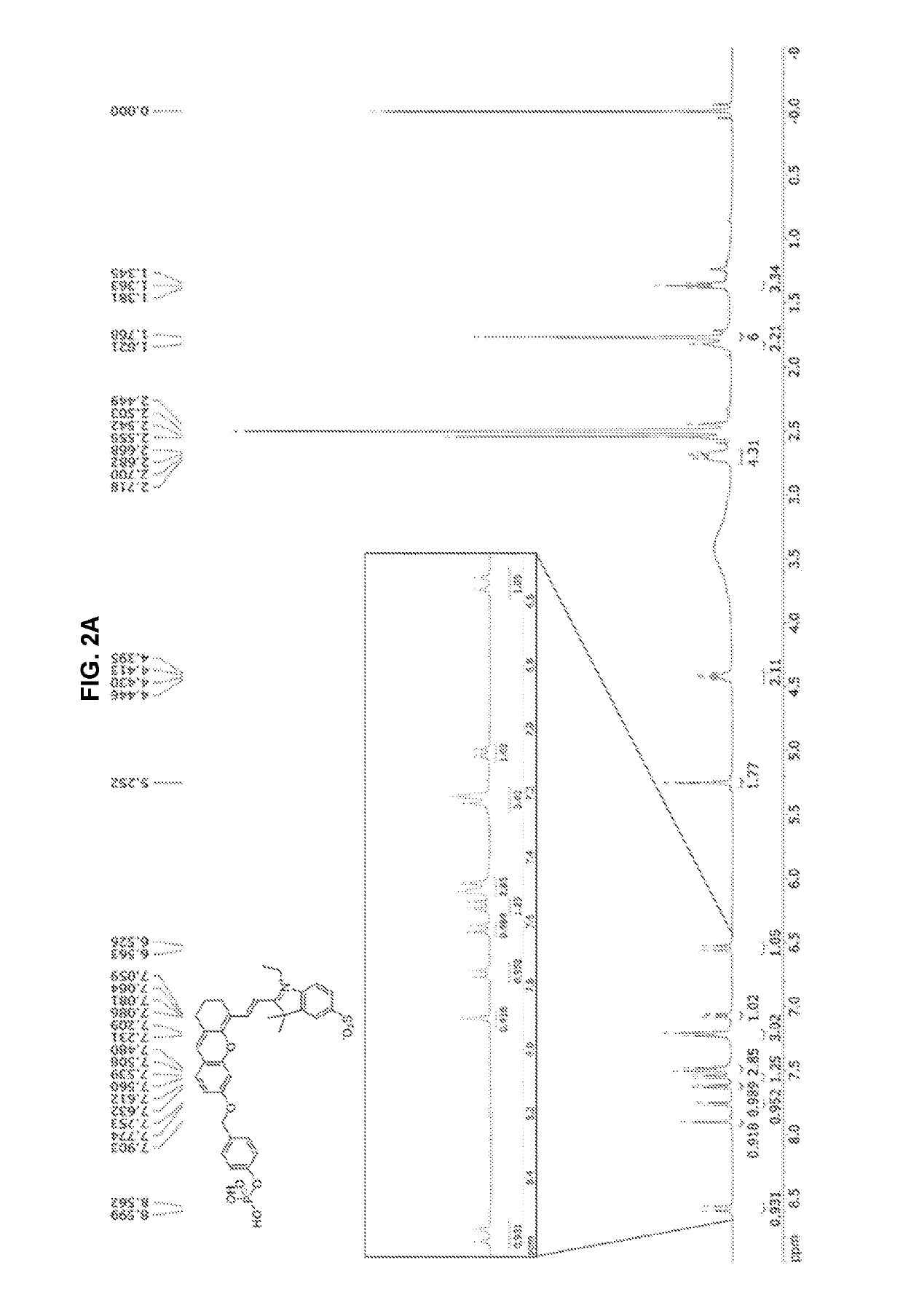
synthesis example 1
nd Represented by Chemical Formula 15
[0103]Generally known 4-(((tert-butyldimethylsilyl)oxy)methyl)phenol (compound represented by Chemical Formula 12) was prepared.
[0104]Then, dichloromethane (DCM, 100 mL) was mixed with a mixture of the prepared 4-(((tert-butyldimethylsilyl)oxy)methyl)phenol (3.84 g, 16.1 mmol) and trimethylamine (TEA, 11 mL / 80.5 mmol) and then diethyl chlorophosphate (5.56 g, 32.2 mmol) was added. Then, the reaction mixture was stirred overnight at room temperature under argon atmosphere. The reaction mixture was diluted with DCM (100 mL), extracted with water (2×100 mL) and then washed with brine (100 mL). Then, the organic layer was dried with Na2SO4 and concentrated under reduced pressure to obtain an oily crude product (compound represented by Chemical Formula 14). The crude product was used in the following process without further purification.
[0105]The crude product obtained above was stirred together with EtOH (100 mL). Then, after adding HCl (8 mL) at roo...
synthesis example 2
nd Represented by Chemical Formula 5
[0110]Dichloromethane (DCM, 57 mL) was mixed with a mixture containing the prepared compound represented by Chemical Formula 15 (1.2 g, 4.61 mmol) and carbon tetrabromide (2.29 g, 6.92 mmol) and then triphenylphosphine (1.81 g, 6.92 mmol) was added at 0° C. Then, the reaction mixture was stirred at room temperature for 7 hours.
[0111]After removing the solvent by evaporating, the reaction mixture was purified by silica column chromatography using ethyl acetate / n-hexane (volume ratio: 1 / 2 to 1 / 1) as an eluent.
[0112]The result of 1H NMR and 13C{1H} NMR spectroscopy and mass spectroscopy of the finally obtained compound represented by Chemical Formula 5 ((4-(bromomethyl)phenyl diethyl phosphate), 1.3 g, 87%) is as follows.
[0113]1H NMR (CDCl3, 600 MHz): δ 7.38 (d, 2H, J=9.0 Hz), 7.20 (d, 2H, J=8.4 Hz), 4.48 (s, 2H), 4.24 (m, 4H), 1.37 (m, 6H) ppm.
[0114]13C{1H} NMR (CDCl3, 120 MHz): δ 150.67, 150.63, 134.46, 130.51, 120.30, 120.27, 64.70, 64.65, 32.68, ...
synthesis example 3
nd Represented by Chemical Formula 4
[0116]Generally known 6-methoxy-2,3-dihydro-1H-xanthene-4-carbaldehyde was prepared.
[0117]After dissolving the 6-methoxy-2,3-dihydro-1H-xanthene-4-carbaldehyde (200 mg, 0.82 mmol) in DCM (8.3 mL), a 1 M BBr3 DCM solution (20 eq, 16.5 mL, 16.5 mmol) was added at 0° C. and the mixture was stirred at 25° C. for 16 hours. Then, the mixture was added to a NaHCO3 solution at 0° C. and the aqueous layer was extracted with DCM / MeOH (10:1 mixture, 2×50 mL). Then, the organic layer was washed with H2O and dried with Na2SO4. Then, after removing the solvent, the product was dried in vacuo to finally obtain a compound represented by Chemical Formula 4 (6-hydroxy-2,3-dihydro-1H-xanthene-4-carbaldehyde) as yellow solid (184 mg, 97%).
[0118]The result of 1H NMR spectroscopy and mass spectroscopy of the finally obtained compound is as follows.
[0119]1H NMR (DMSO-d6, 600 MHz): δ 10.18 (s, 1H), 7.19 (d, 1H, J=9.0 Hz), 6.92 (s, 1H), 6.61 (sd, 1H, J=2.4 Hz), 6.59 (dd, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelengths | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com