Anti-HER2 glycoantibodies and uses thereof
a glycoantibody and anti-her2 technology, applied in the field of therapeutic monoclonal antibodies, can solve the problems of reducing the life span of the circulatory system of the drug, affecting the effector function of the therapeutic antibody, and provoking immunogenicity, so as to improve the therapeutic value, increase the binding affinity of the fc receptor, and increase the activity of the adcc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation of Exemplary Anti-HER2 GAbs
Anti-HER2 GAb301
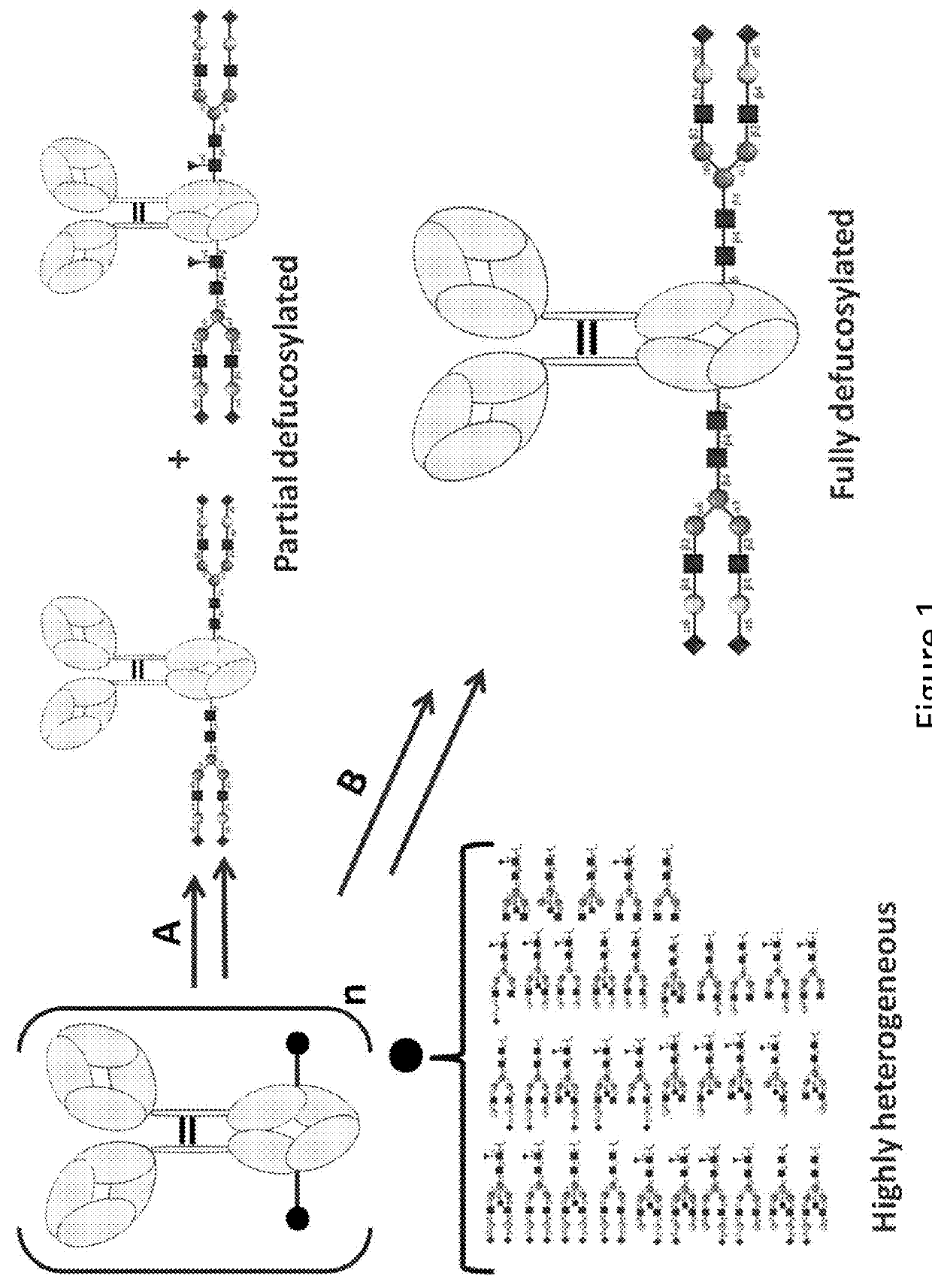
[0235]The complete removal of N-linked glycan at Asn297 from Fc region of Trastuzumab (Herceptin) is achieved by means of PNGase F, and evaluated with 4-12% Bis-Tris NeuPAGE and LC-MS / MS analysis of tryptic glycopeptides from modified and unmodified IgG. The molecular weights of tryptic glycopeptides helps to determine the potential site of N-linked glycosylation at each asparagine and to elucidate the species of predominant glycans.
Anti-HER2 GAb201
[0236]A glycoantibody derived from conventional Trastuzumab (Herceptin) bearing a glycan structure of GlcNAc-Fuc in the Fc region was treated with one or more endoglycosidases (Endo S and / or Endo F1, Endo F2, Endo F3), and then treated with an alpha-fucosidase to cleave the core fucose with a high cleavage efficiency and lead to a glycoantibody with a monosaccharide GlcNAc in the Fc region.
[0237]Mixtures of Endo S (10 μg), Endo F1 / Endo F2 / Endo F3 (10 μg), Fucosidase (2 mg) and Trastuzu...
example 2
Characterization of Anti-HER2 GAbs
General
[0261]MS spectrometry analysis of of glycoengineered mAb. Intact molecular weights of glycoengineered antibodies were detected by LC-ESI-MS on a LTQ Orbitrap XL ETD mass spectrometer (Thermo Fisher Scientific, San Jose, Calif.) equipped with ACQUITY UPLC (Waters, Milford, Mass.), using a BEH C4 column (1.0 mm×150 mm, 1.7 □m, Waters, Milford, Mass.). Briefly, the gradient employed was 1% buffer B at 3 min to 40% buffer B at 20 min with a flow rate of 50 uL / min, where buffer A was 0.1% formic acid in H2O and buffer B was 0.1% formic acid in 80% acetonitrile. Full-scan MS condition: mass range m / z 800-4000 with Iontrap. Electrospray voltage was maintained at 4.0 kV and capillary temperature was set at 275° C.
[0262]For the analysis of trypsinized glycopeptides, high resolution and high mass accuracy nanoflow LC-MS / MS experiments were done on a LTQFT Ultra (Linear quadrupole ion trap Fourier transform ion cyclotron resonance) mass spectrometer (Th...
example 3
Anti-HER2 GAb ELISA Binding Assay
General
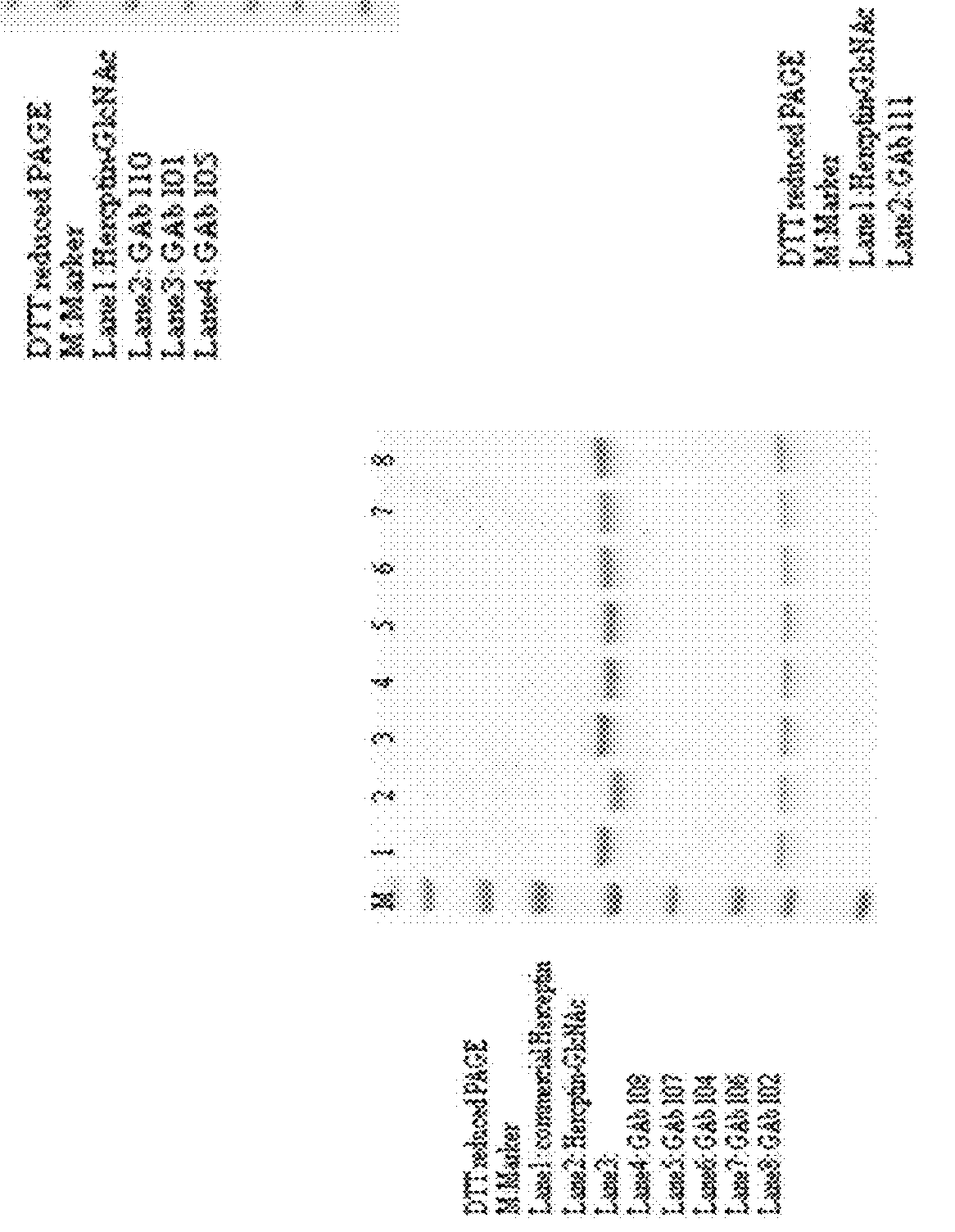
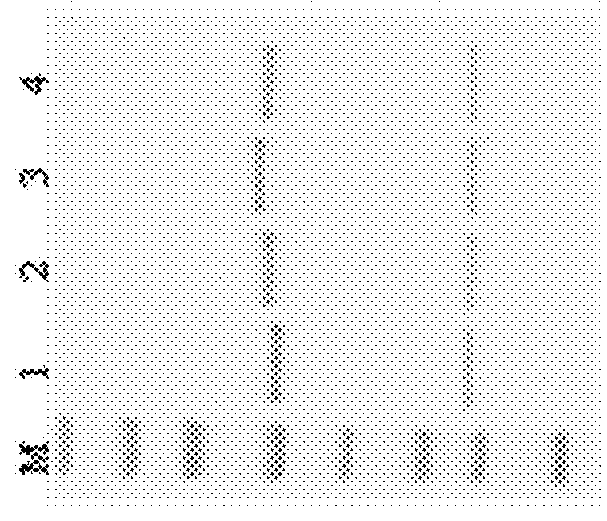
[0272]SDS-PAGE detection of glycoengineered Herceptin antibodies. All the SDS-PAGE analysis was performed with NuPAGE® Novex® 4-12% Bis-Tris gel (Invitrogen) ether with or without DTT addition.
[0273]Recombinant human HER2 protein (purchased from Sino Biological Inc.) (1 μg / mL) in 100 μL carbonate coating buffer (pH 10) was coated onto 96 well high binding plates at 4° C. for overnight. 2% of bovine serum albumin in PBST was then added to block plates at r.t. for 1 hr and subsequently a series dilution of anti-HER2 GAbs 101, 102, 104, 105, 106, 107, 108, 110 and 111 were added to plates and incubated for one hour. After incubation, HRP conjugated anti-human IgG was added to the reaction mixture and incubated for one hour. An aliquot of OPD substrate was added, and the absorbance at 450 nm was recorded. Throughout the experiment, plates were washed with PBS twice after each incubation step. Results of ELISA binding are shown in Table 8.
[0274]
TAB...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com