(2-alkoxy-4-oxo)-penta-2-enoate compound preparation method
An alkoxy and alkenoate technology, which is applied in the field of preparation of -pent-2-enoate compounds, can solve the problems of equipment corrosion, waste acid water generation, complicated post-treatment, etc., and achieves mild reaction conditions and environmental protection. The effect of less pollution and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
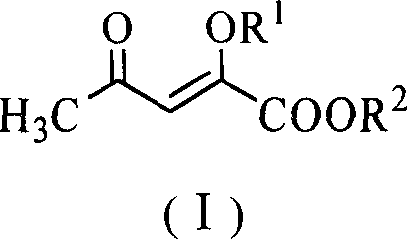
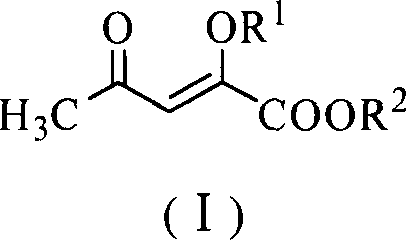
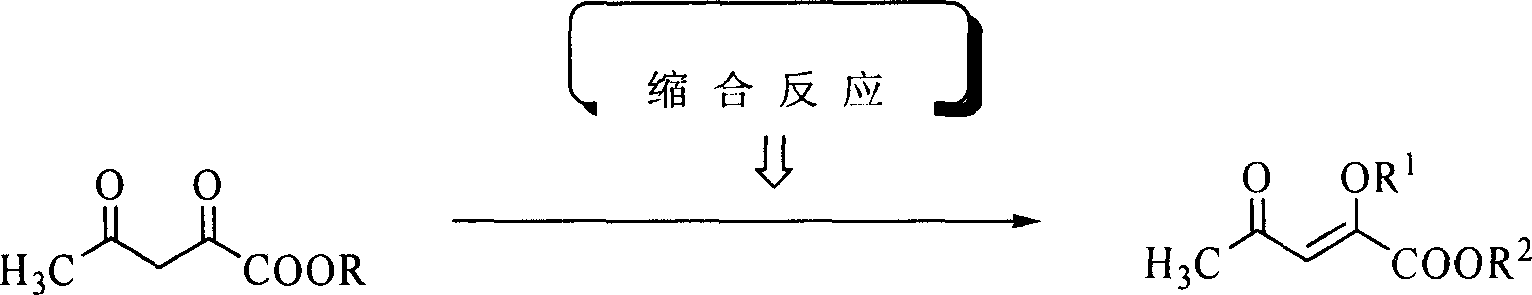
[0020] Embodiment 1: Preparation of (2-methoxy-4-oxo)-pent-2-enoic acid methyl ester (I)
[0021] Methyl acetylacetonate (14.4 g, 0.1 mol), trimethyl orthoformate (11.7 g, 0.11 mol), polystyrene-supported ferric chloride (6.8 g, 0.01 mol) and anhydrous methanol (25 mL) were placed Dry the reaction bottle and stir it well at 45°C for 5h. After the reaction is complete, cool to room temperature, filter, recover the catalyst, and recover methanol from the filtrate under reduced pressure. Add ethyl acetate (150mL) to the residue, continue to stir for 5min, let it stand, separate the organic layer, and wash with 10% sodium carbonate solution (20mL×5), water (20mL×3), saturated saline (20mL×3) successively 2) Wash and dry over anhydrous magnesium sulfate. After recovering the solvent, distill under reduced pressure and collect fractions at 107-109°C / 13mmHg to obtain light yellow oil I (R 1 =R 2 =CH 3 , 13.4g, 85%). The purity is 98.2% (GC-MS).
[0022] 1 H NMR (CDCl 3 ): δ=...
Embodiment 2
[0024] Embodiment 2: Preparation of (2-methoxy-4-oxo)-pent-2-enoic acid methyl ester (I)
[0025] Methyl acetylacetonate (14.4 g, 0.1 mol), trimethyl orthoformate (31.9 g, 0.3 mol), polystyrene-supported titanium tetrachloride (139.8 g, 0.2 mol) and anhydrous methanol (50 mL) were placed Dry the reaction bottle and stir it well at 60°C for 2h. After the reaction is complete, cool to room temperature, filter, recover the catalyst, and recover methanol from the filtrate under reduced pressure. Add ethyl acetate (200mL) to the residue, continue stirring for 5min, let it stand, separate the organic layer, and wash with 10% sodium carbonate solution (30mL×5), water (30mL×3), saturated saline (30mL×3) successively 2) Wash and dry over anhydrous magnesium sulfate. After recovering the solvent, distill under reduced pressure, collect 107-109 ℃ / 13mmHg fraction, obtain light yellow oily product I (R 1 =R 2 =CH 3 , 14.2g, 90%). The purity is 98% (GC-MS). 1 Both HNMR and MS are con...
Embodiment 3
[0026] Embodiment 3: Preparation of (2-ethoxyl-4-oxo)-pent-2-enoic acid ethyl ester (I)
[0027] Ethyl acetylacetonate (15.8 g, 0.1 mol), triethyl orthoformate (16.3 g, 0.11 mol), polystyrene-loaded ferric chloride (6.8 g, 0.01 mol) and absolute ethanol (25 mL) were placed Dry the reaction bottle and stir it well at 45°C for 5h. After the reaction is complete, cool to room temperature, filter, recover the catalyst, and recover methanol from the filtrate under reduced pressure. Add ethyl acetate (150mL) to the residue, continue to stir for 5min, let it stand, separate the organic layer, and wash with 10% sodium carbonate solution (20mL×5), water (20mL×3), saturated saline (20mL×3) successively 2) Wash and dry over anhydrous magnesium sulfate. After recovering the solvent, distill under reduced pressure, collect 127-129 ° C / 11mmHg fractions, and obtain light yellow oily substance I (R 1 =R 2 =C 2 h 5 , 16.7g, 90%). The purity is 98.2%. (GC-MS).
[0028] 1 H NMR (CDCl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com