Preparing process for Isolation of a plurality of isoflavones components in astragalus root
A technology of isoflavones and astragalus, applied in the field of formononetin, can solve the problems of large solvent consumption, difficult separation and purification, complex reaction products, etc., and achieve the effect of good reproducibility, simple solvent system and large separation volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
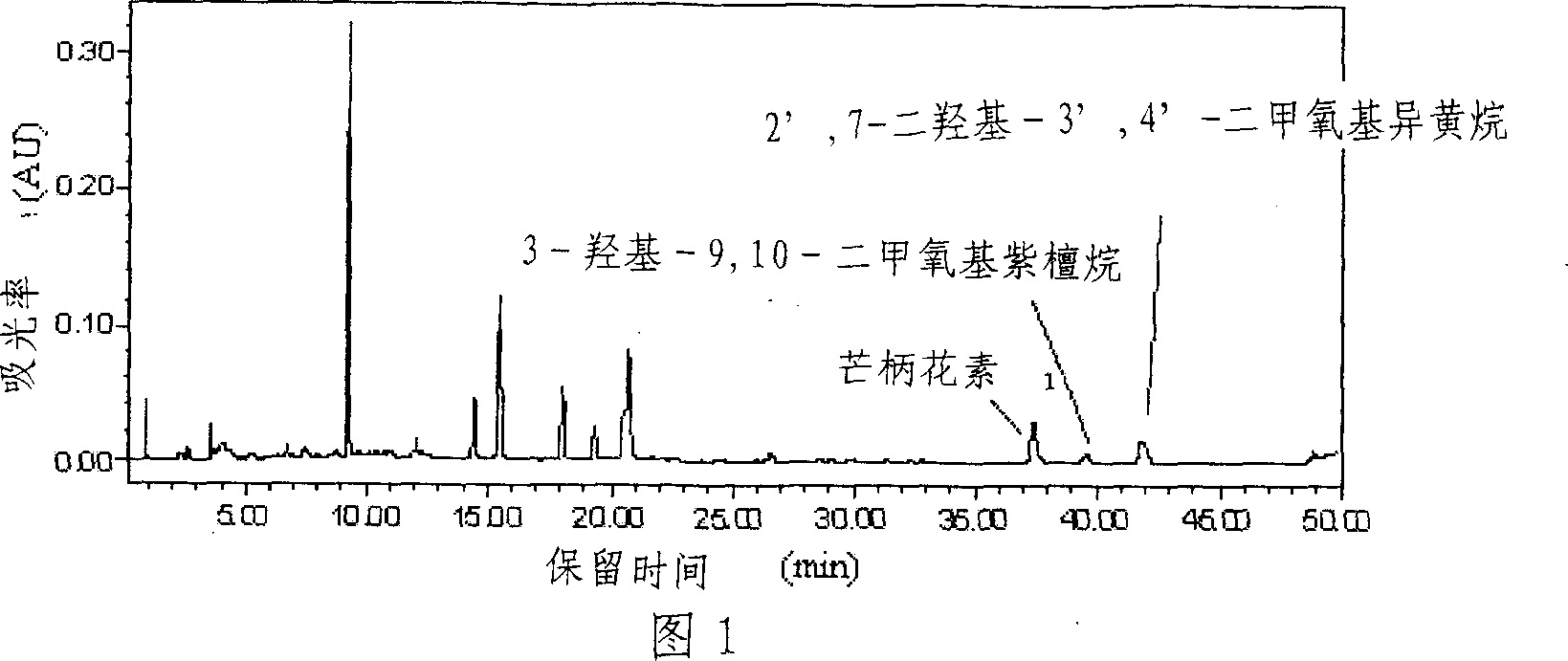
[0021] 1. Weigh 20 g of the crude extract of Astragalus total flavonoids (see Figure 1 for HPLC analysis), add methanol to dissolve it, mix the sample with silica gel at a mass ratio of 1:2 to 3 (w / w), and mix After the sample is uniform, evaporate the solvent and grind evenly.
[0022] 2. Use 100-200 mesh silica gel (1300g) to fill the chromatography column and perform column chromatography. The elution solvent is petroleum ether-ethyl acetate (1:1 by volume) and eluted with 4 times the column volume.
[0023] 3. the outflow part detects with thin-layer chromatography, developing agent is sherwood oil-ethyl acetate (volume ratio is 1: 1), and chromogen is the methanol solution containing 5% sulfuric acid, collects and contains target product (R f ≥0.62), the fractions containing formononetin, 9,10-dimethoxypurantane and 2',7-dihydroxy-3',4'-dimethoxyisoflavane were combined into a group Divided into Fr2, concentrated into extract 757mg. High-performance liquid chromatograph...
Embodiment 2
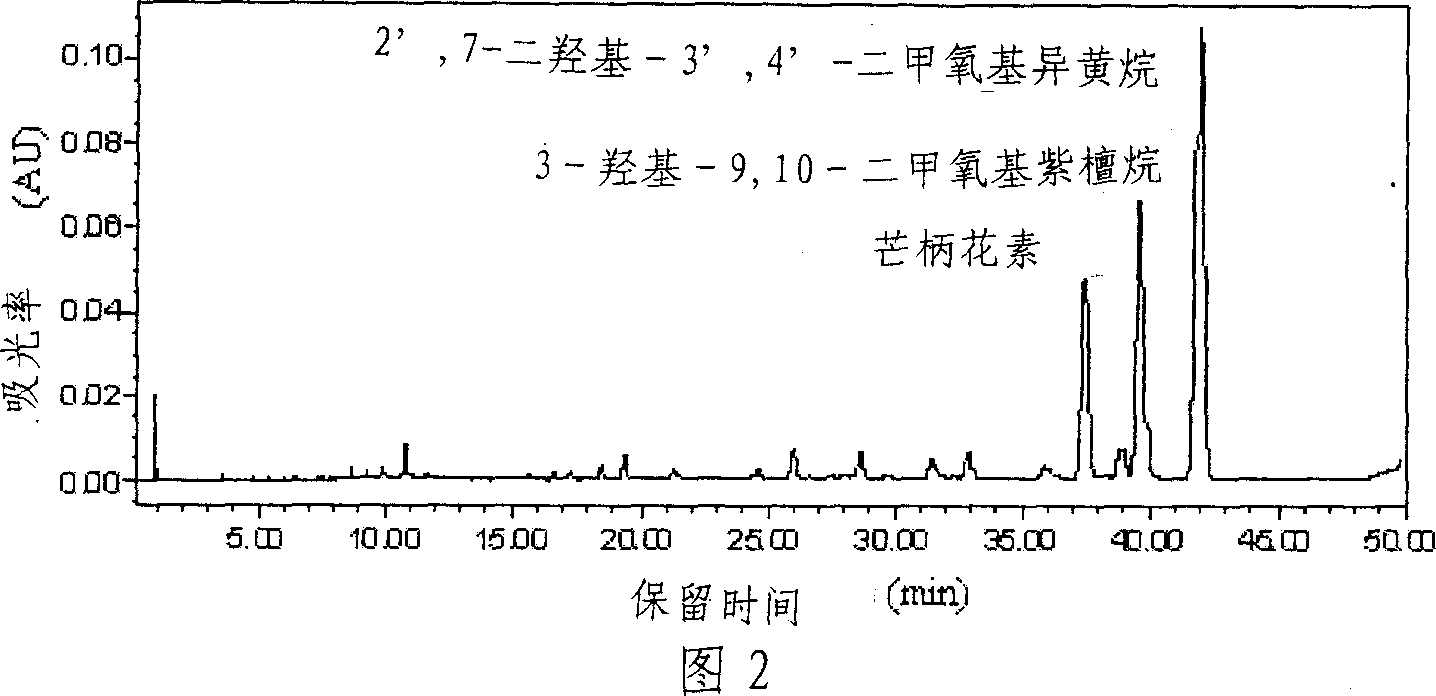
[0031] The preparation mode of component Fr2 is the same as embodiment 1. 287.5 mg of component Fr2 was weighed and dissolved in 10 mL of methanol-chloroform (volume ratio 1:2) solution system, and passed through a 0.45 μm filter membrane as a preparative chromatographic injection solution. The inner diameter of the preparative chromatographic column is 10 cm, the column length is 20 cm, the column filler is C18 (particle size 10 μm), and the injection volume is 20 mL. Inject the sample into the injection loop of the preparative chromatography, use methanol and 0.5% formic acid as the eluting phase, the volume ratio of the two-phase solvents is 50:50, carry out isocratic elution at a flow rate of 300mL / min, and run for 55min . On-line monitoring of the fractions by a UV detector, the detection wavelength is 230nm, cut according to the peak and collect the effluent to obtain 96 mg of formononetin, 41.6 mg of 9,10-dimethoxy pterocarpine, 2',7-dihydroxy-3 ', 4'-Dimethoxyisoflav...
Embodiment 3
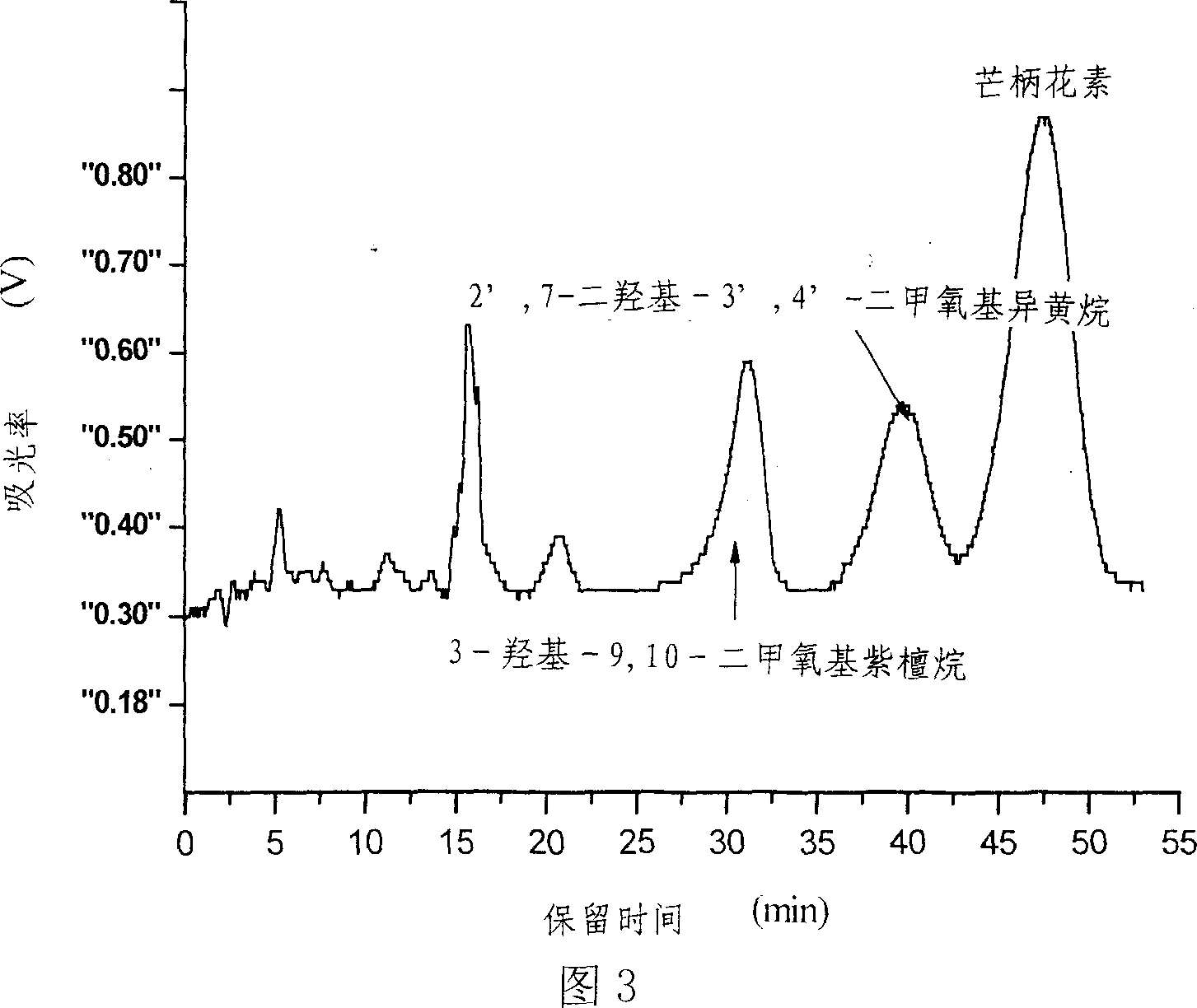
[0033] The preparation mode of component Fr2 is the same as embodiment 1. 11 mg of component Fr2 was weighed and dissolved in 5 mL of methanol-chloroform (volume ratio 1:2) solution system, and passed through a 0.45 μm filter membrane to prepare a chromatographic injection solution. The inner diameter of the preparative chromatographic column is 2 cm, the column length is 25 cm, the column filler is C18 (particle size 10 μm), and the injection loop is 10 mL. Inject the sample into the injection loop of the preparative chromatography, use methanol and an aqueous solution containing 0.5% formic acid as the eluting phase, carry out isocratic elution with the volume fraction of the two-phase solvent at 50:50, the flow rate is 19mL / min, and the running time is 55min . The fraction was monitored by an online UV detector, the detection wavelength was 230nm, the effluent was cut according to the peak and the effluent was collected to obtain 3.9mg of formononetin, 1.76mg of 9,10-dimet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com