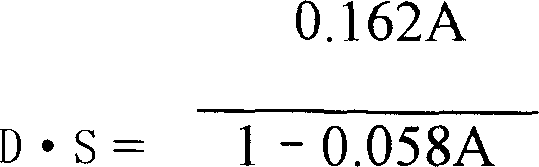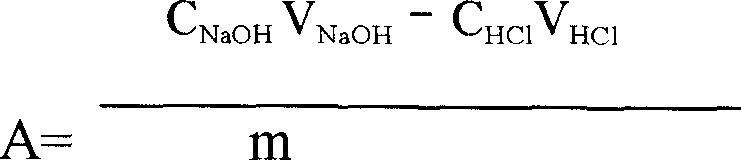High substitution degree carboxymethyl indianbread polysaccharide and its preparation method and uses
A Poria cocos polysaccharide technology with a high degree of substitution is applied to metabolic diseases, medical formulas, medical preparations of non-active ingredients, etc. It can solve the problems of limiting the clinical application range of CMP, difficult separation and removal of impurities, and difficult industrial production, etc. Environmental protection, shortening of reaction time, and improvement of solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1 Preparation of CMP with high degree of substitution
[0093] High degree of substitution CMP is prepared by the following method:
[0094] 1) 75 kg of dried poria cocos, crushed into fine powder, put into a soaking tank, pumped into 400L of water, stirred evenly, and soaked for 12 hours;
[0095] 2) Slowly pump 400L of sodium hydroxide solution with a concentration of 2.25mol / L into the soaking tank, stir and react for 1h, filter, and take the filtrate;
[0096]3) Add 300 L of chloroacetic acid solution with a concentration of 5.3 mol / L into the neutralization tank, then slowly add 300 L of sodium hydroxide solution with a concentration of 6.25 mol / L, stir until the reaction is sufficient, and cool to room temperature;
[0097] 4) Pump the reaction solution in step 3) into the filtrate obtained in step 2), mix well, then slowly raise the temperature to 75°C, and react at constant temperature for 2.5 hours;
[0098] 5) Use 6mol / L hydrochloric acid solutio...
Embodiment 2
[0111] Example 2 Determination of Molecular Weight of Carboxymethyl Pachyranan
[0112] Determination conditions: the instrument is LKB column chromatography system, SephadexG-200 column. The mobile phase is 0.2mol / L NaCl solution (pH 7.0) buffer solution, the flow rate is 0.5ml / min, the injection volume (W / V) is 1mg / ml, and the constant temperature is 10°C. Differential refraction is automatically detected, and the recorder records the peak position.
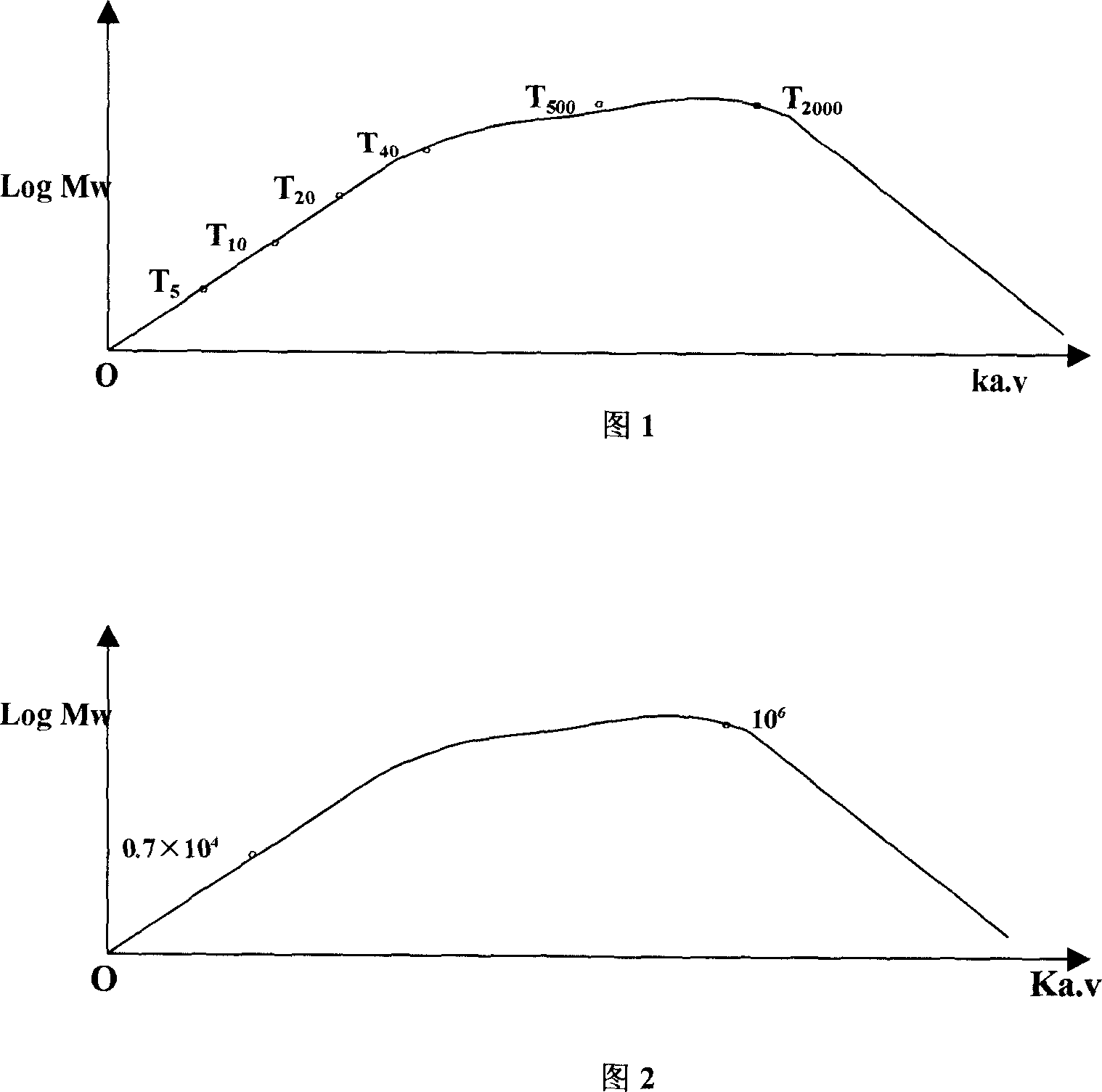
[0113] Standard curve: use standard polysaccharides Dexfran T2000 (Mw2000000), T500 (Mw500000), T40 (Mw40000), T20 (Mw20000), T10 (Mw10000), T5 (Mw5000) with known molecular weight to make a standard curve, according to Ka.v logarithm Log Mw plotted, the standard curve is shown in Figure 1.
[0114] The elution curve of the carboxymethylpachyran that the present invention makes on the SephadexG-200 chromatographic column is referring to Fig. 2, according to the Ka.v value that records, obtains its molecular weight (Mw) to...
Embodiment 3
[0116] Example 3 Determination of Carboxymethyl Substitution Degree (D·S) of Carboxymethylpachyan by Acidification Method
[0117] Precisely weigh 0.4041g of carboxymethylpachyran sample, put it in a 150ml beaker, heat in a water bath at 80°C, stir to dissolve, after cooling, adjust the pH to 4.0 with 2mol / L hydrochloric acid solution, add 100ml of absolute ethanol, stir Evenly, let it stand overnight, centrifuge to separate the alcoholate, and wash the alcoholate repeatedly with 95% ethanol until the washing liquid does not contain chloride ions. Wash the alcohol analyte and dissolve it with 40.00ml of 0.1000mol / L NaoH standard solution. After the solution becomes transparent, immediately use 0.1000mol / L standard hydrochloric acid solution for back titration until the red color of the phenolphthalein indicator just fades away, record the back titration The volume of the consumed 0.1000mol / L hydrochloric acid standard solution is 21.60ml.
[0118] Calculate the degree of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com