Near infrared boron fluoride complexed dipyrrolyl methine fluorescent dye for biological analysis
A technology of dipyrrole methine and fluorescent dyes, applied in the field of fluorescent dyes, can solve the problems of unsatisfactory degree of red shift, poor photostability, and poor conjugation of phenyl and naphthyl groups with dye precursors, and achieves protonation process. reversible effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
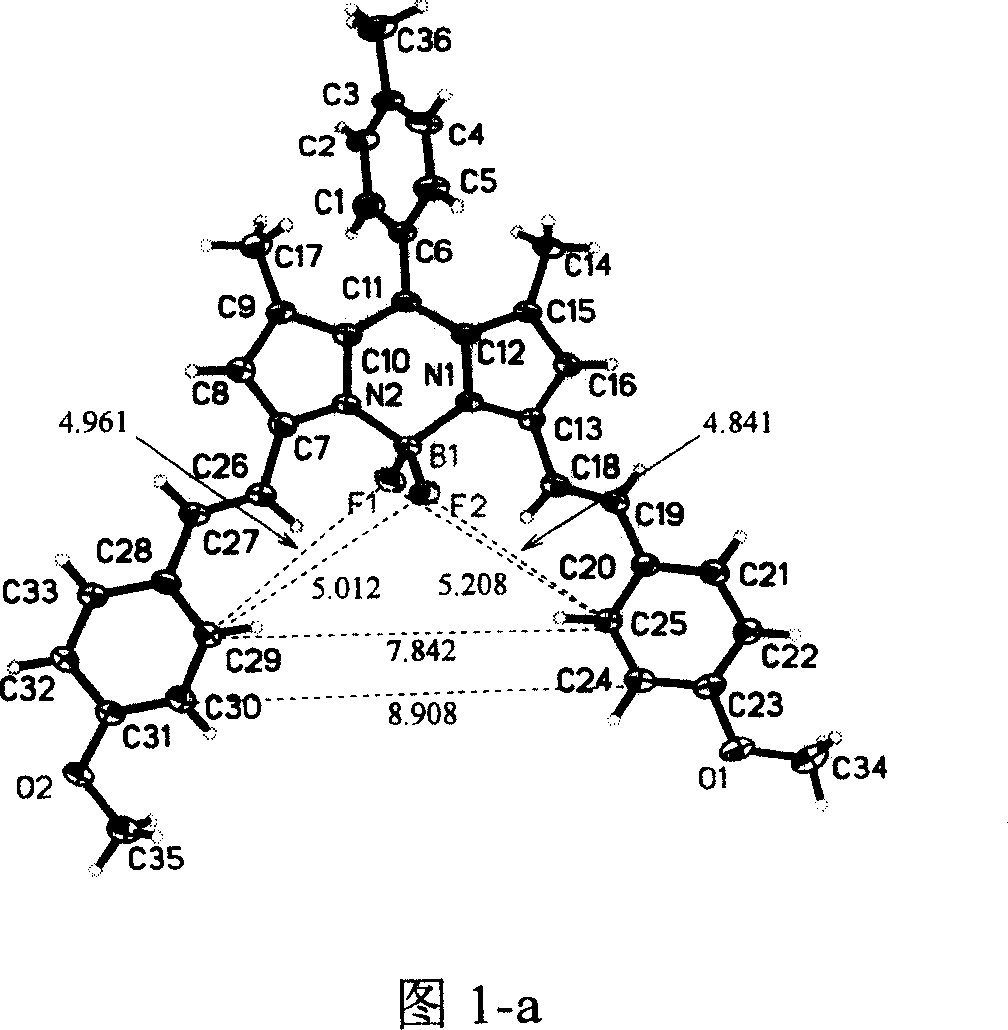
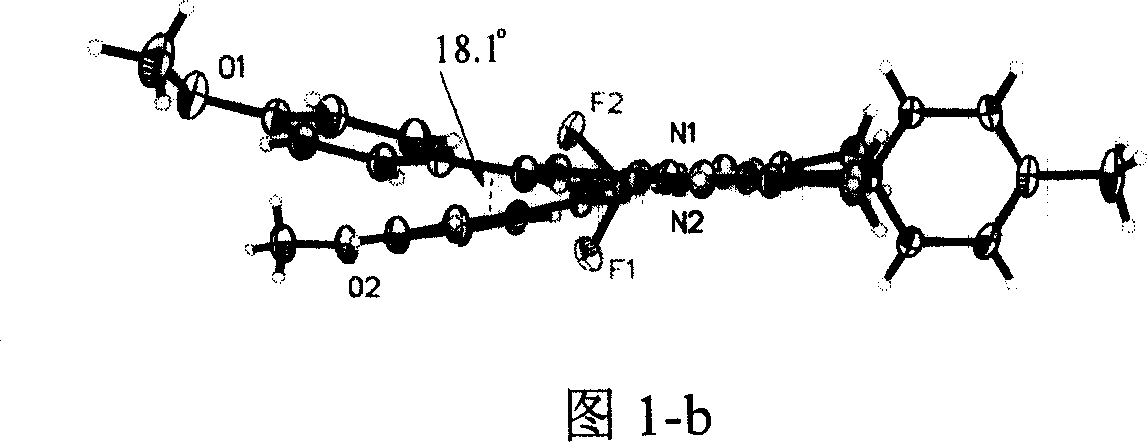
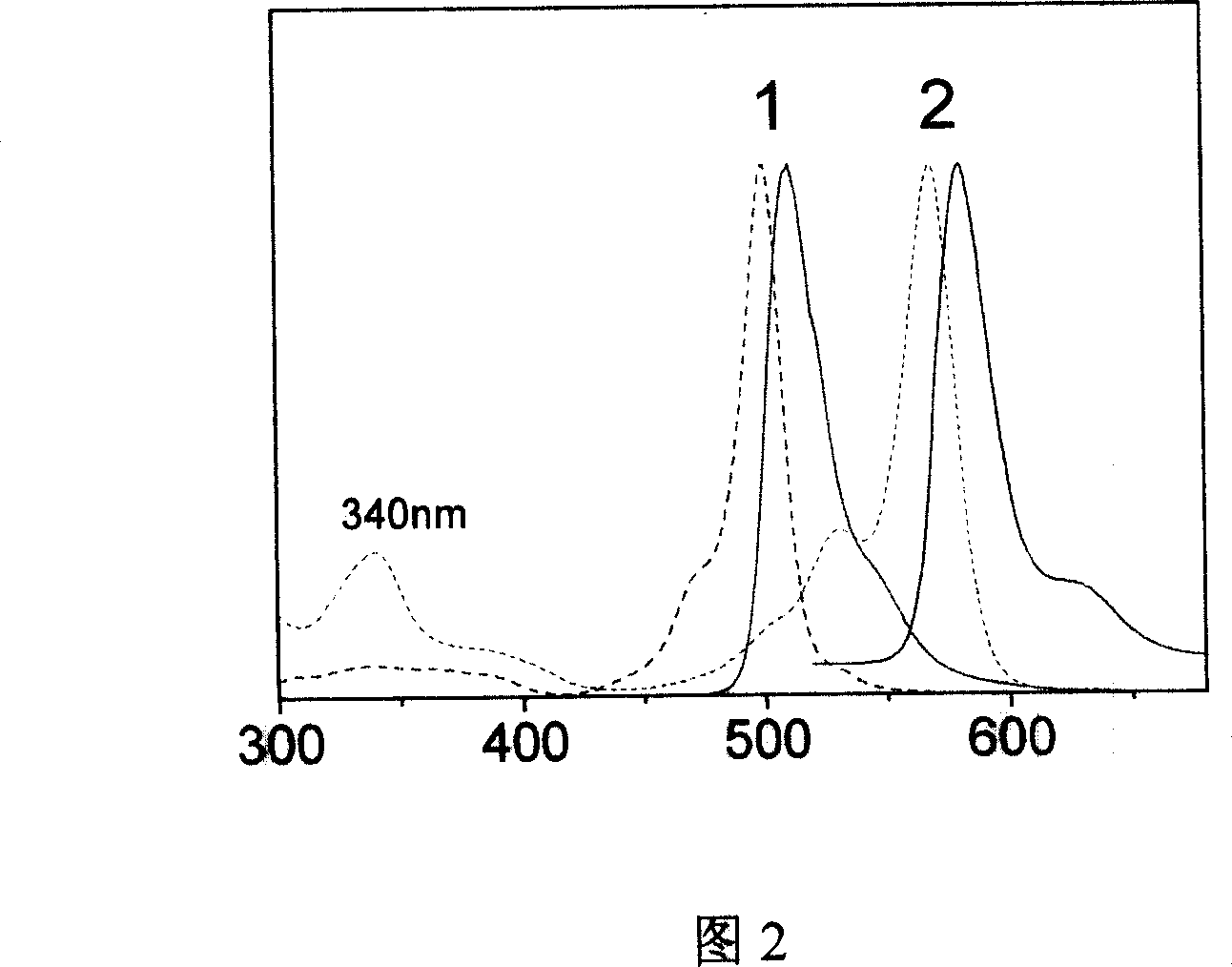
Image
Examples
Embodiment 1
[0072] The synthetic route of intermediate 2,4-dimethylpyrrole:
[0073]
[0074] Take 120ml of ethyl acetoacetate and 400ml of glacial acetic acid, add them to a 1000ml three-necked flask, control the temperature below 25°C, and stir mechanically. Weigh 100g of sodium nitrite and dissolve it in 120ml of water to make an aqueous solution. Use a dropping funnel to slowly add it dropwise to the above-mentioned mixed solution. After 4 hours of dripping, the temperature is controlled at room temperature and stirred for 5 hours. Weigh 136g zinc powder, heat the temperature of the above reaction solution to 60℃, gradually add zinc powder, and at the same time measure 136ml ethyl acetoacetate slowly dropwise into a three-necked flask with a dropping funnel, the dropwise addition is completed within two hours, the reaction time Heat, produce red nitrogen dioxide gas, while flocculent solids appear in the reaction liquid, the solids gradually increase, and finally can not stir completely...
Embodiment 2
[0077] R 1 Synthesis and characterization of the parent dye that is methyl:
[0078]
[0079] The synthesis of the parent dye can be carried out continuously in a one-pot reaction, and the intermediate product does not need to be separated and purified. The operation steps are as follows: Take a 500mL single-mouth flask, dissolve 2mmol 2,4-dimethylpyrrole and 1mmol p-methylbenzaldehyde in 200mL dichloromethane, replace the air in the flask with argon, and bubbling with argon The air in the solution was driven out, and then a drop of trifluoroacetic acid left catalyst was added dropwise. The reaction system was continuously stirred under the protection of argon, and the reaction temperature was room temperature (25°C). The reaction was terminated after 2 hours. The reaction solution was washed twice with 100 mL of 0.1 mol / L NaOH solution, then the organic layer was dried with anhydrous sodium sulfate, and the solvent methylene chloride was distilled off under argon protection, an...
Embodiment 3
[0081] Synthesis of compound IIa:
[0082]
[0083] 0.1mmol (approximately 33.8mg) of the dried parent compound (product of Example 2) was added to a 25ml round-bottomed flask, all dissolved with 10ml of anhydrous o-dichlorobenzene, and 5 dried molecular sieves were added, using a micro-injector 0.12 mmol (about 14.4 mg) of p-tolualdehyde purified by distillation was added dropwise, and finally 0.1 ml of piperidine was added as a catalyst. The entire reaction system was stirred uniformly under the protection of argon, heated to 120°C and continued to react for 10 hours, and the progress of the reaction was tracked by TLC every 3 hours, and the fluorescence intensity was observed under a 365nm ultraviolet lamp until there was no more The product appeared. The reaction system was gradually cooled to room temperature, the reaction mixture was diluted with 50 ml of dichloromethane, washed twice with 40 ml of water, the organic layer was dried with anhydrous sodium sulfate, concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com