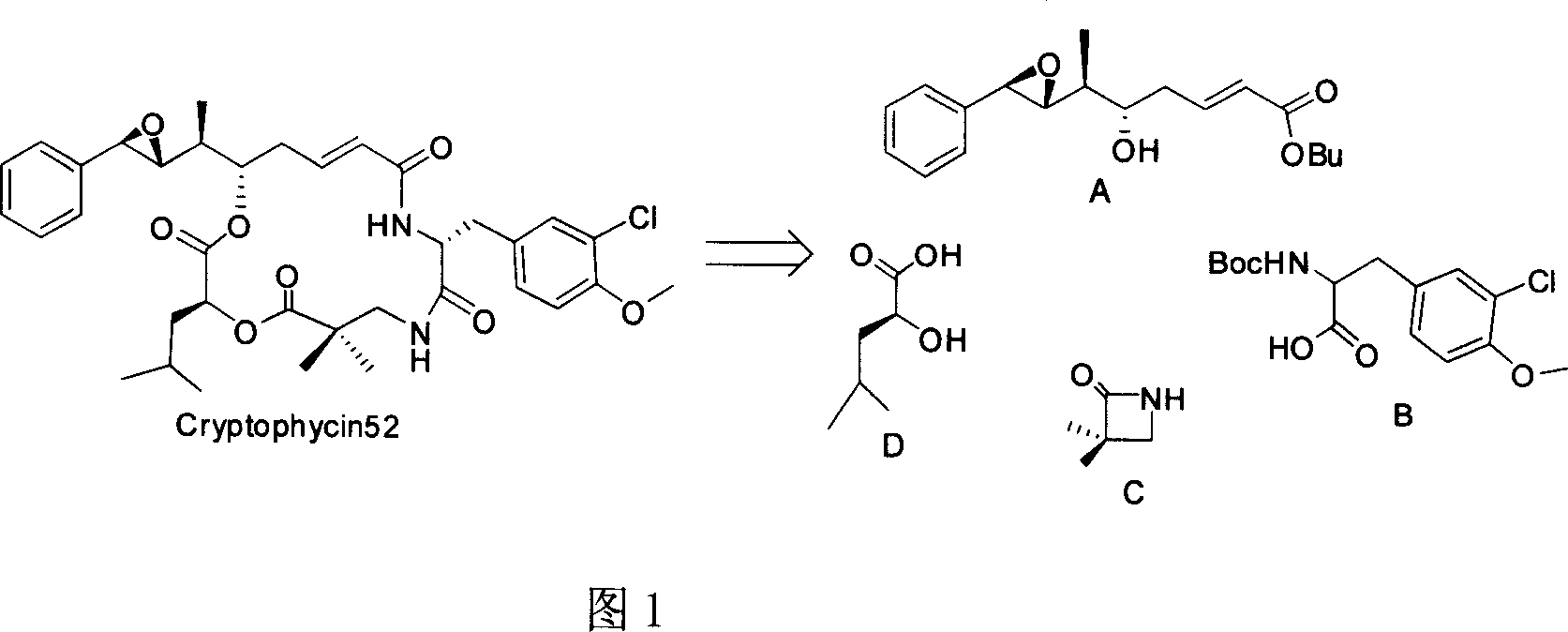
Anti-cancer active compound depside peptide as derivative of natural product
A technology of depsipeptides and natural products, applied in the field of medical engineering, can solve the problems of poor stability, environmental sensitivity, poor water solubility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
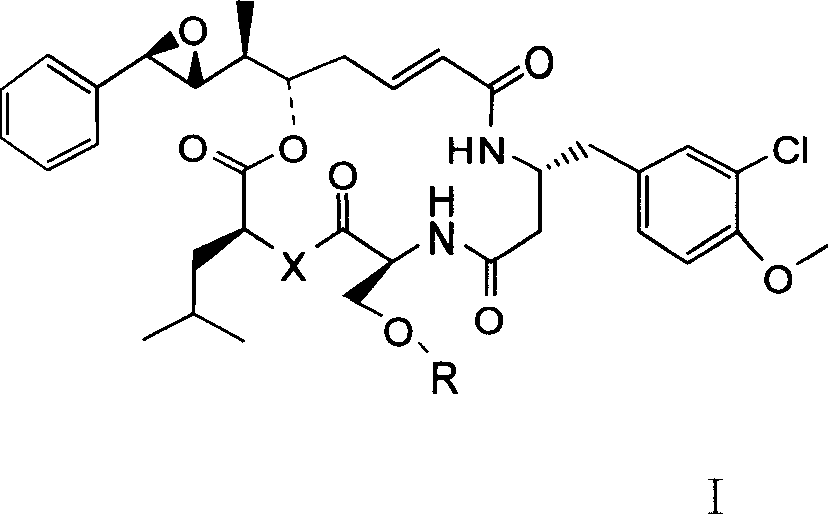
[0032] Total synthesis method: (taking X=O, R=H, Y=NH in CryptophycinsIV series as an example)
[0033] 1. Fragment A (pre-synthesis 1.0eq), fragment D (L-leucic acid TBS protected hydroxyl 1.2eq), add DCC (2.eq), DMAP (cat), CH2Cl2 as solvent; after mixing, stir at room temperature for 6 hours, Silica gel column separation, obtain product (67%)
[0034] 2. The product (1.0eq) obtained in 1 and C fragment (CSI: chlorosulfonyl isocyanate, 1.2eq) were mixed in an ice-water bath at 0°C. After 30 minutes, the solvent was evaporated to dryness under reduced pressure and used directly in the next reaction. Fragment D: (R)-tert-butyl 1-amino-3-(3-chloro-4-methoxyphenyl)propyl-2-ylcarbamate (1.3eq) was added to anhydrous CH2Cl2 of the previous product , Et3N (3.0 eq) was added, followed by stirring at room temperature for 2 hours. The product was isolated on a silica gel column (65%).
[0035] 3. Add 2 product to TFA, CH 2 Cl 2 After stirring at room temperature overnight, the pr...
Embodiment 2
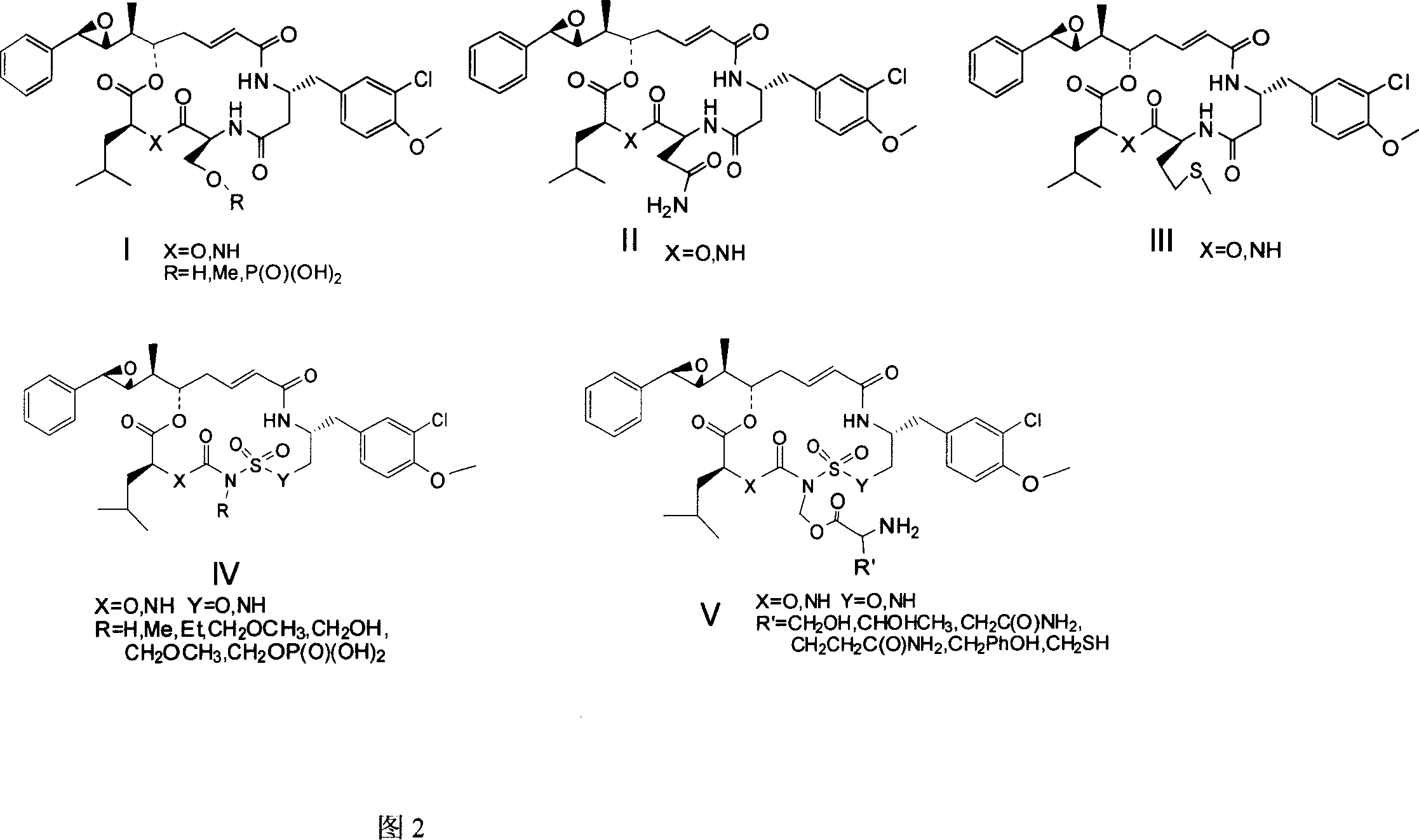
[0038]Other derivatives of the CryptophycinsIV series are all prepared by the method 1.2.3 in the above-mentioned embodiment 2, except that the conversion of the 4 and 8-position O and N atoms is completed when the D and B fragments are synthesized, and the others are all modified after the synthesis of the macrocycle. Corresponding methylation, ethylation, hydroxymethylation, methoxymethylation, and phosphorylation after hydroxymethylation are carried out on the 6-position N atom to obtain respective corresponding derivatives.
[0039] The NMR data of methylation products are as follows:
[0040] 1 HNMR (CDCl 3 ): δ7.39(m, 5H), 7.20(S, 1H), 7.08(d, J=8.4Hz, 1H), 6.88(d, J=8.4Hz, 1H), 6.59(m, 1H), 6.02 (d, J=7.5Hz, 1H), 5.77(d, J=15.7Hz, 1H), 5.65(m, 1H), 5.10(m, 2H), 4.20(m, 1H), 3.90(s, 3H) , 3.72(s, 1H), 3.38(s, 3H), 3.31(m, 2H), 2.96(d, J=7.0Hz, 1H), 2.89(m, 1H), 2.78(m, 1H), 2.57( m, 1H), 2.41(m, 1H), 1.90(m, 1H), 1.75(m, 2H), 1.43(m, 1H), 1.18(d, J=7.0Hz, 3H), 0.91(m...
Embodiment 3
[0043] The derivatives of Cryptophycins I, II, and III series were also prepared by the method 1.2.3 in Example 2 above to complete the total synthesis of 16-membered macrocycles. The difference in the molecule is that the conversion of the 4-position O and N atoms is completed when the D segment is synthesized, and the main difference between the derivatives of the Cryptophycins I, II, and III series is the difference in the C segment. In the derivatives of the Cryptophycins I series, the C fragment is L-serine, and the hydroxyl group of the hydroxymethyl group on the 6-position C is further methylated and phosphorylated to obtain each corresponding derivative.
[0044] The C-segment of the derivatives of Cryptophycins II series is L-asmandoamide.
[0045] The C fragment of the derivative of Cryptophycins III series is L-methionine.
[0046] The above-synthesized Cryptophycins series of compounds can be used to conduct research on their anti-cancer activity and study their a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com