Method for inspecting hepatitis and AIDS virus nucleic acid by synchronous amplification and its reagent kit
An HIV, kit technology, applied in biochemical equipment and methods, microbial determination/inspection, etc., to avoid missed detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
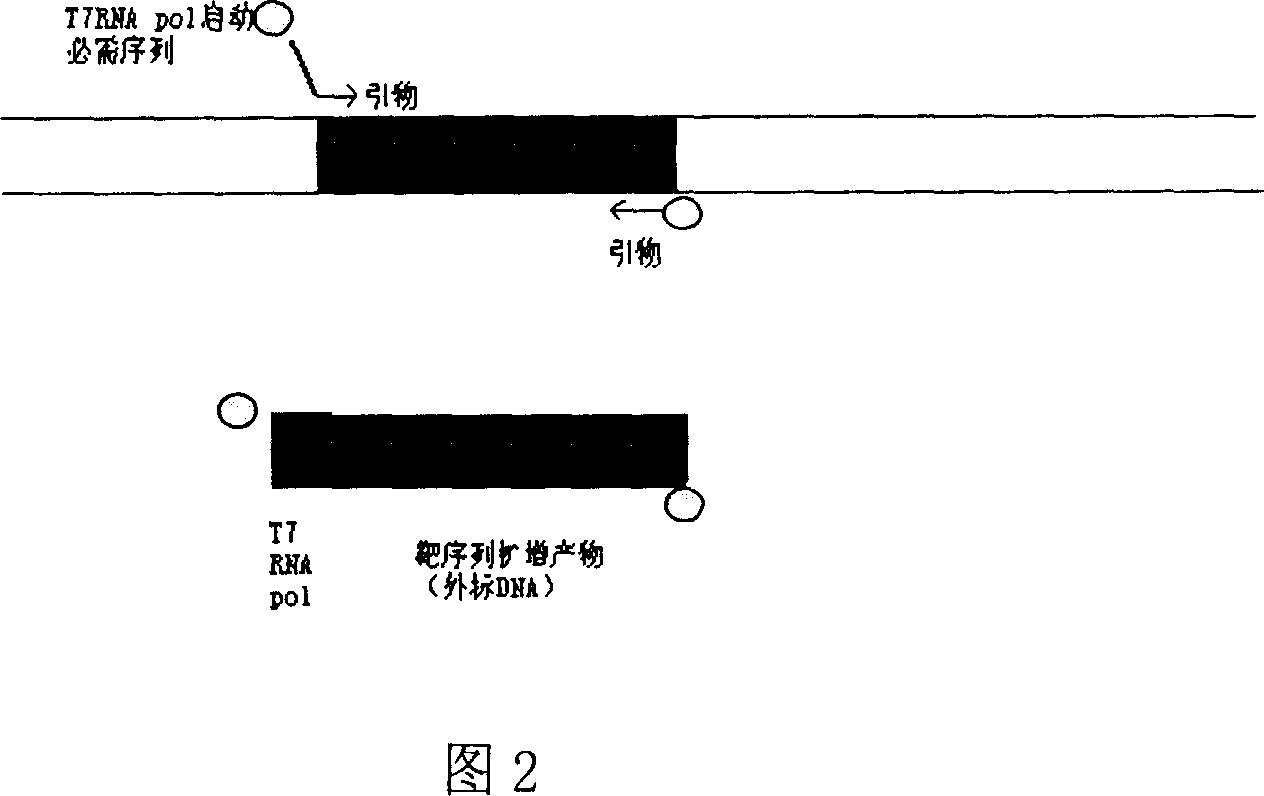
[0044] Example 1 Rapid preparation of internal control and positive control by biotin-modified primer plus terminal PCR method (the basis of the green environmental protection kit),
[0045] 1. The preparation of HIV internal control and positive control is as follows
[0046] 1.1 Rapid construction of positive control: commission Shanghai Sangon to synthesize the following primers, the sequence is:
[0047] 5’BIOTIN-AAT TCT AAT ACG ACT CAC TAT AGG GAG gac atc aag cag cca tgc aaa t-3’
[0048] (The italic part at the 5' end is the T7 RNA pol promoter region, and the 3' end is the upstream primer of the HIV GAG region)
[0049] (SEQ.ID.NO.10)
[0050] 5'BIOTIN-cta tgt cac ttc ccc ttg gtt ctc tc-3' (downstream primer of HIV GAG region)
[0051] (SEQ.ID.NO.11)
[0052] Take one specimen (or one plasmid containing HIV GAG gene) that is clinically positive for HIV nucleic acid, and...
Embodiment 2
[0113] Example 2 Specimen processing unit: magnetic bead method for nucleic acid extraction and reagent preparation
[0114] The reagent composition of the specimen processing unit based on the principle of hybridization includes:
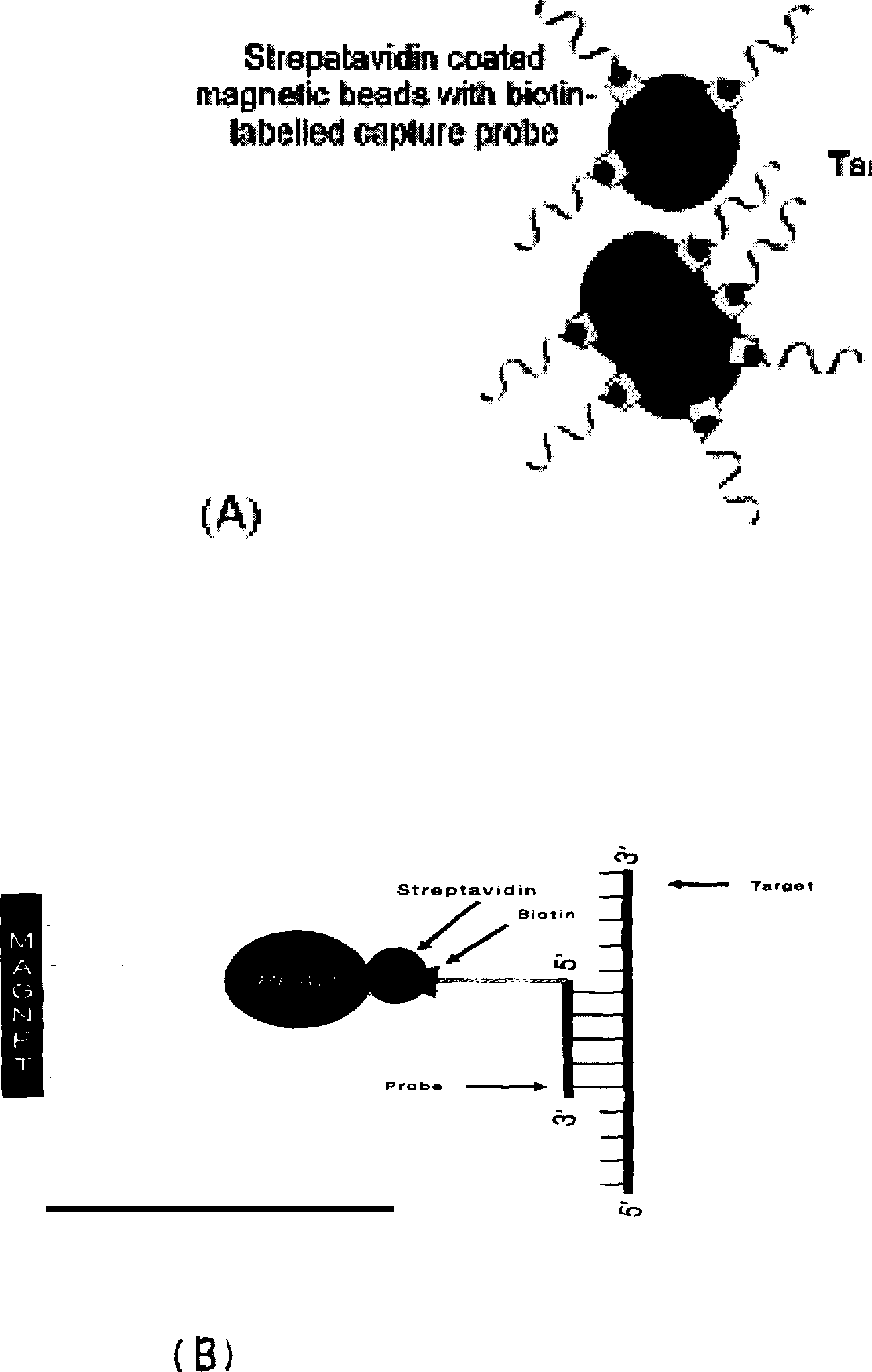
[0115] Lysis solution: 4.8M guanidine thiocyanate, 50mM Tris, PH7.0, NP-40, Triton X-100, SDS, biotinylated probe, polymer, etc. where the capture probes are:
[0116] 5'BIOTIN-acc acc aaa tgc ccc tat-3'(HBV)
[0117] 5'BIOTIN-agt acc aca agg cct ttc g-3'(HCV)
[0118] 5'BIOTIN-cta tgt cac ttc ccc ttg gtt ctc tc-3'(HIV)
[0119] Magnetic bead suspension: 1mg / ml streptavidin-coated magnetic beads, containing preservatives and dispersants such as Tween 20
[0120] Washing liquid A: LiCI, Tris PH7.0, lithium dodecylsulfonate, preservatives and dispersants such as Tween 20, pigments, etc.
[0121] Washing liquid B: LiCI, Tris PH7.0, preservatives and dispersants such as Tween 20, pigments, etc.
[0122] Washing liquid C: LiCI, Tris PH7.0, preservat...
Embodiment 3
[0134] Example 3 Nucleic acid amplification detection unit: single-tube dual-enzyme one-step RT-PCR TaqMan probe multi-detection system mode and reagent preparation
[0135] The nucleic acid amplification detection unit adopts fluorescent TaqMan PCR, which is required to be used with a fluorescent PCR instrument. Its components include the following:
[0136] RT-PCR reaction solution: buffer containing 1.8Mm d NTPs (including d UTP), 3.6mM DTT, 3.5mM MgCl2, primers BF, BR, CF, CR, IF, IR 0.4μM each.
[0137] Enzyme mixture: 0.6U / μl AMV reverse transcriptase, 0.6U / μl hot-start Taq DNA polymerase, 1U / μl RNasin; 0.1U / μl UNG (heat-labile), etc.
[0138] Probe: 10μM probe HBV Probe, HCV Probe, HIV Probe, 5μM internal standard probe, stabilizer and buffer.
[0139] Reagent ratio and preparation: According to the ratio of RT-PCR reaction liquid:enzyme mixture:probe=8:6:1 for each test, 15 μl / test is prepared, and 15 μl of nucleic acid template is added to the machine for amplificat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com